Introduction
Retentive filters designed to provide effective and consistent clearance of parvovirus (∼20 nm) have now become a standard in downstream purification processes for biologics manufactured in mammalian cells. These retentive filters are also commonly known as small virus filters in relation to those filters designed for removing larger viruses such as retroviruses. There are currently several brands of small virus filters that vary in chemical composition and structural configuration depending on individual manufacturers. These differences translate to variations in flux, matrix sensitivity, tolerance of virus preparations, and operating pressures. Despite these differences, all of these filters retain viruses primarily by the mechanism of size exclusion (1, 2, 3). It has become increasingly clear that small virus filters can consistently provide reliable and effective removal of larger viruses such as retroviruses (1, 4, 5). Consequently, it is logical to claim retrovirus clearance based on the studies carried out with parvoviruses. Depending on the quality of virus preparations used, filter performance measured by throughput as measured by liters per square meters of filter per hour (L/m2/h) can be significantly affected. The importance of virus preparation quality has now been broadly recognized (6, 7). While virus-retentive filtration is considered as the most desirable dedicated virus clearance unit operation for its robustness in virus removal and absence of adverse impacts on product quality, parvovirus breakthrough continues to be a common phenomenon and factors that influence the breakthrough and/or filter performance remain not well understood (3).
In this session, speakers from 11 companies presented their research/development work and shared experience/learning in the area of virus filtration.
Proposed Operation and Validation of Multiple Viral Filtration Cycles within a Harvest Lot (Eva Gefroh, and Megan McClure; Amgen)
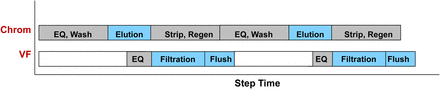
In the purification of an entire harvest lot of product, multiple cycles of chromatography are typically used to minimize equipment size and resin costs. Process intermediate hold tank volume constraints may preclude the pooling of all elution cycles, and instead each cycle may be forward-processed to the next unit operation. This would result in cycling of the viral filter along with the chromatography step, as illustrated in Figure 1. A method was presented in which the viral filter was cycled within a harvest lot, with multiple cycles of alternating product load and buffer flush steps. In this proposed method, the viral filter is still validated to the total product and buffer flush loading from all cycles, and a post-use integrity test is performed after all cycles are completed. Because the subsequent viral filter cycle may not be performed immediately following the prior cycle, the viral filter could experience a static hold condition between cycles.
Illustration of a chromatography elution pool that is forward-processed to the viral filtration step over two cycles. EQ = Equilibration; Regen.= Regeneration.
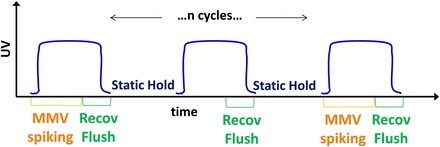
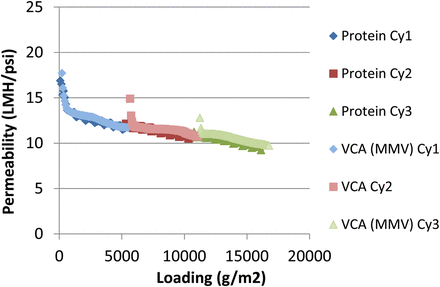
The introduction of discretized cycles within the total loading of the viral filter allows for an alternative approach to be considered for viral validation. Similar to the approach for validating a chromatography resin, in which the resin is validated at the first cycle (new resin) and last cycle (used resin), the virus retention on the filter could likewise be assessed as a bracketing of the first and last cycles. This proposed viral validation strategy is shown in Figure 2, with the overall step clearance given by the average clearance from the first and last cycles. The testing of the last cycle would represent worst-case retention due to pore plugging of the filter at the maximum expected loading. Testing of the first cycle would not only provide a baseline for the retentive capabilities of the virus filter at the start of the process, but also represent worst-case retention if caking fouling on the filter surface were to develop, which could result in higher retention factors with increased extent of loading. An example dataset is shown in Figure 3, in which the hydraulic permeability profiles of a protein-only run is compared to a run spiked with mouse minute virus (MMV) at cycles 1 and 3 and only product for cycle 2. The filter was loaded to over 15 kg/m2, with little difference in permeability between spiked and non-spiked runs. The log reduction value (LRV) of cycle 1 was ≥4.84, and ≥4.82 for cycle 3, for an average process LRV of ≥ 4.83.
Proposed bracketed viral clearance strategy for viral filter cycling.
Permeability vs loading for a protein-only run compared to a bracketed MMV-spiked run. (VCA = Viral Clearance Assessment, Cy = Cycle).
In summary, a method has been presented on the operation and validation of multiple viral filtration cycles within a harvest lot. The viral clearance strategy utilizes a bracketed approach to spiking the first and last cycle, and the feasibility of this approach was demonstrated. In this method, the use of a single virus filter for an entire harvest batch provides a clear advantage in minimizing the risk of bioburden contamination by maintaining a closed system. This cycling approach could theoretically be extended to include NaOH sanitization between cycles for compatible filters as a way to further mitigate bioburden contamination. This will be evaluated in future work.
Virus-Retentive Filtration (Thomas Kreil; Global Pathogen Safety—Baxter BioScience)
While virus filtration has been widely used in industry, the potential impact of pressure fluctuations/interruptions on virus retention has only more recently become apparent. An advanced, automated, down-scale system has been developed that allows for the investigation of such phenomena.
In a study that investigated MMV removal by virus filtration with a process that feeds up to 20,000 L intermediate per square meter of virus filter area and filtration times of up to 1 month, including up to 20 pressure interruptions of 1 h to 3 days duration, virus removal of >5 log10 has been obtained. Using the advanced virus filtration down-scale system, critical process parameters were controlled, which resulted in constant pressure that enabled this large-throughput application; also, all process parameters were automatically recorded in compliance with 21 CFR part 11 (8).
Introduction of an Alternate Viral Filter to a Commercial Process and Optimization of Scale-down Filter Capacity (Philippe de Vilmorin; Biogen Idec)
Biogen Idec successfully introduced a new viral filter to a commercial antibody manufacturing process and received approval for the new process with an option to use either of the two viral filters. Viral filters are now dual-sourced for the purpose of supply chain risk mitigation. The new filter significantly reduced process time in manufacturing, and it was validated to provide ≥4.0 log10 clearance for all four viruses tested. In the first viral clearance study, a filter loading of only 300 L/m2 was achieved. The capacity of the viral filter was limited by the purity of the viral stocks and the suboptimal handling of the feed material. The viral clearance study was repeated using high-purity virus stocks and improved handling procedures. The second viral clearance study demonstrated robust viral clearance at >900 L/m2. Implementation of reduced filtration area is expected to yield filter cost savings of >$1 million per year.
Parvovirus Clearance on a Virus-Retentive Filter—Consideration of Log Reduction and Virus Breakthrough in Determining Virus Load Challenge (Eileen Wilson; GlaxoSmithKline)
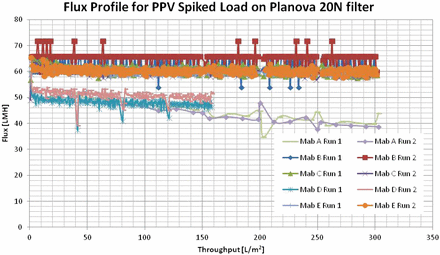
Reduction of porcine parvovirus (PPV) across a Planova 20N filter was determined for five monoclonal antibodies (MAbs) as outlined in Table I. The effect of virus load challenge on virus breakthrough and the resulting LRV was reviewed to determine optimal load challenge. For all experiments (total of 13 PPV spiked experiments for five MAbs), PPV-spiked load was processed through a 0.001 m2 Planova 20N filter at 14 psi constant pressure at ambient room temperature. Four filtrate fractions were collected, followed by a buffer flush of 5 to 15 mL. The small-scale model was operated with a brief pressure release after load completion to allow for flush buffer addition to the reservoir. There was not much flux decay during the filtrations, as shown in Figure 4.
Buffer Matrixes of MAb Load Materials
Flux profiles of PPV spiked load on Planova 20N filter.
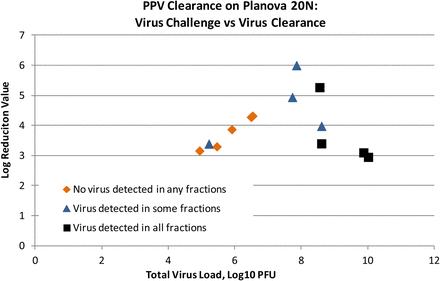
The cumulative LRV was calculated by summing the virus measured in individual fractions and comparing to the total virus in the load. As shown in Figure 5, experiments with a load of >7 log10 total plaque-forming units (PFU) (>10 log10 PFU/m2 filter area) consistently showed breakthrough in some or all fractions. The LRV is reported as a greater-than value for experiments with no virus detected or with virus detected in some, but not all, fractions. Load challenge for PPV on 0.001 m2 Planova 20N filter will target 8 log10 total PFU for future studies to maximize LRV.
Parvovirus clearance by Planova 20N: virus load vs LRV achieved, illustrating the observation that parvovirus breakthrough escalates as parvovirus load increase, resulting lower LRV.
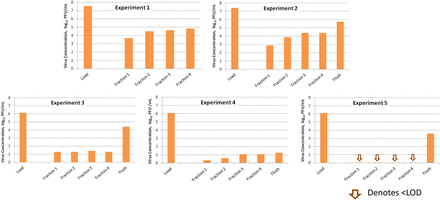
Breakthrough was detected with higher virus challenge, but the pattern of breakthrough was not consistent. In five experiments with the same MAb in the same matrix at the same volumetric load ratio, the distribution of virus in the fractions varied, as shown in Figure 6. Experiments 3, 4, and 5 had a similar total virus challenge, but Experiments 3 and 5 showed higher virus concentration in the flush than in any of the filtrate fractions while Experiment 4 did not. The resulting LRVs for Experiments 3, 4, and 5 were 3.40, 5.27, and >3.98, respectively.
PPV detected in filtrate fractions from five individual experiments. Data from five experiments with the same MAb in the same matrix at the same volumetric throughput are shown. Experiments 1 and 2 had higher virus load, and virus breakthrough was detected in all factions as well as buffer flush permeate. Experiments 3, 4, and 5 had similar virus load (lower than Experiments 1 and 2), yet virus breakthrough pattern varied. Fraction 1: up to 150 L/m2; Fraction 2: 150 to 200 L/m2; Fraction 3: 200 to 250 L/m2; Fraction 4: 250 to 300 L/m2; Flush: 6–11 mL (flush not tested separately for Experiment 1).
Low Pressure and High Membrane Load Lead to Virus Breakthrough in Virus Removal Filtration of a MAb (Susan Liu; Janssen Research and Development)
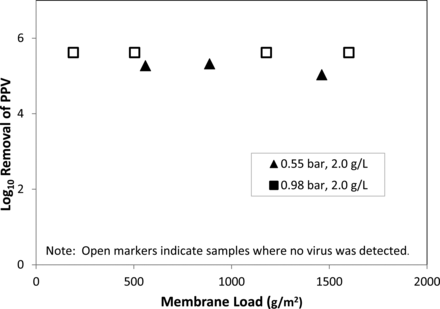
Janssen presented PPV clearance studies that were performed to assess the limits of a pressure-fed virus filtration system used for the purification of a therapeutic MAb. The PPV clearance results are presented as a function of membrane load (g/m2) and filtration pressure (Figure 7). In the discussed runs, PPV-spiked load material at 2 g/L was filtered at differential pressures of 0.55 and 0.98 bar. Fractions were collected and assayed for titers over a range of membrane loads. Janssen observed no viral breakthrough at 0.98 bar (indicated by the open squares) but observed viral breakthrough at 0.55 bar (indicated by closed triangles). This observation was consistent over the range of membrane loads tested.
Removal of PPV as a function of membrane load and filtration pressure.
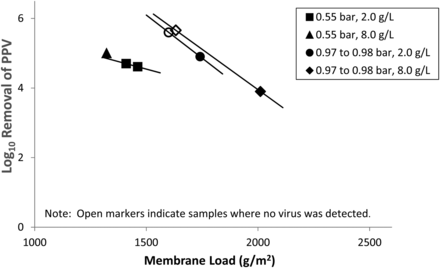
Additional data was presented to demonstrate the relationship of PPV clearance with filtration pressure, membrane load, and protein concentration (Figure 8). In runs performed at 0.55 bar (closed squares and triangle), breakthrough was observed and PPV clearance appeared to decrease with increasing membrane load. At 0.98 bar, no breakthrough was observed at membrane loads ∼1600 g/m2 (open circle and diamond), but as membrane loads approached 2000 g/m2, breakthrough occurred (closed circle and diamond). PPV clearance appeared to decrease with increasing membrane load. At both low and high filtration pressures, viral clearance was independent of protein concentration.
PPV clearance as a function of membrane load, filtration pressure, and load protein concentration (data provided by Andrew Arbutina, Terry Benner, Jesse Richter, Alison Harkins, Gloria Kim, and Susan M. Liu).
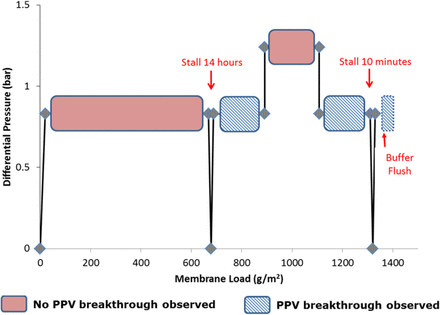
The results of a “surge and stall” study were also presented to demonstrate the effects of variable pressure on viral clearance (Figure 9). The study conditions were as follows: filtration at 0.8 bar (0 to 700 g/m2), stall at 0 bar for 14 h, filtration at 0.8 bar (700 to 900 g/m2), pressure increase followed by filtration at 1.2 bar (900 to 1100 g/m2), pressure decrease followed by filtration at 0.8 bar (1100 to 1300 g/m2), filter stall at 0 bar for 10 min, buffer flush at 0.8 bar. Sample fractions where PPV breakthrough was detected are indicated by hatched rectangles in Figure 9. Virus was detected in three collected fractions: following the 14 h stall, following the sudden decrease in pressure from 1.2 to 0.8 bar, and the buffer flush pool following the 10 min stall. Virus breakthrough occurred after sudden decreases in pressure (following a significant pressure decrease or filter stall). A minimum of 3.5 LRV was demonstrated for all fractions in the study.
Occurrence of PPV breakthrough as a function of filtration pressure, membrane load, and filtration stall time (data provided by Terry Benner, Andrew Arbutina, and Susan M. Liu).
In conclusion, Janssen observed that for its viral filtration system, PPV breakthrough is more likely to occur at low filtration pressure and high membrane load. In a pressure surge and stall study, virus breakthrough occurred after sudden decreases in filtration pressure.
Effect of pH, Conductivity, Pressure Release on Parvovirus Clearance (Sean O'Donnell, Adith Venkiteshwaran, and Dayue Chen; Eli Lilly and Company)
Eli Lilly and Company reported the influence of operating conditions on PPV retention by viral filtration. Several parameters were examined; these included (i) pressure, (ii) depressurization/repressurization (DPRP) prior to buffer flushing, (iii) pH, and (iv) conductivity. Lab-scale filtration experiments were carried out at three different pressures (100%, 66%, or 33% of the recommended pressure) for a polyvinylidene fluoride (PVDF) parvovirus filter. Grab samples, collected at the beginning, middle, and end of filtration, as well as filtrate and buffer flush samples were collected to monitor PPV breakthrough by median tissue culture infective dose (TCID50) assay. The results indicate that the effectiveness of PPV removal as measured by log reduction factor (LRF) is dependent on operating pressure for the given brand of the parvovirus filter with low pressure being worse-case. PPV was detected in both filtrate and buffer flush samples from the experiment carried out at 33% pressure. On the other hand, PPV was only detected in the buffer flush at 66% or 100% pressure. Moreover, it was demonstrated that PPV breakthrough seen in buffer flushing was caused by the DPRP cycle and can be prevented or minimized by avoiding DPRP prior to buffer flushing, which can be readily achieved through engineering.
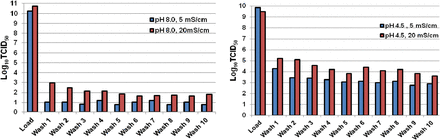
The effect of pH and conductivity on PPV retention during viral filtration was also investigated. More PPV breakthrough was observed at pH 4.5 than at pH 8.0 in buffer flushing after DPRP. Furthermore, conductivity also has an effect on PPV breakthrough during buffer flushing after DPRP, with higher conductivity resulting in more PPV breakthrough as shown in Figure 10. Taken together, our study shows that effectiveness of parvovirus clearance by the filter can be affected by operating pressure, DPRP cycle, and matrixes. Further studies are needed in order to determine whether the observed effects of pH and conductivity are due to electrostatic interactions between viruses and filter membrane or something else.
Influence of pH and conductivity on parvovirus breakthrough.
Impact of Using High-Purity Virus Spikes on the Apparent Breakthrough of MMV during Viral-Retentive Filtration Evaluations and Maintaining Virus Retention during Depressurisation Events (James Berrie; Lonza)
Lonza presented its work on the influence of virus spike preparation on the breakthrough of virus during 20 nm viral-retentive filtration evaluations and how to mitigate potential parvovirus breakthrough following depressurization. Initial in-house data at Lonza suggests that in general, relatively high-purity virus spikes compared with “standard” spikes result in a much greater degree of MMV breakthrough. However, the data indicated that there may be an influence on the degree of MMV breakthrough by other factors such as the conductivity of the protein solution. The phenomenon is further confounded by the data set indicating no MMV breakthrough where no pressure drop was implemented during the spiked runs and the implementation of a buffer flush was achieved in a “coupled” process allowing maintenance of high pressure throughout. During discussion of the data it is apparent that other groups have data to suggest breakthrough of small viruses occurs when pressure halts are implemented for increasing periods of time. In addition, data was discussed (not presented) on the increase in MMV breakthrough for some 20 nm filters when they are operated below a set pressure. Lonza's approach to worst-case filter evaluation, whereby over-capacity and over-pressure are implemented, was discussed in light of these observations. At Lonza, a single depressurization event is generally incorporated, and this occurs between filtration of the product and introduction of the rinse or flush buffer. It has been suggested that pressure perturbations may influence breakthrough of small viruses during the filtration of product (prior to the rinse). The requirement for a pressure interruption where a filter is particularly sensitive to feedstream interruptions in pressure was discussed based upon the example presented, where a “coupled” filtration process was described where no pressure drop occurred (and no MMV breakthrough) in order to avoid fouling of the virus-retentive filter. In such cases a requirement to show pressure drop data could present a challenge. It was concluded that a consideration of what parameters should be included in filter evaluations for studies designed to support early phase clinical trials should be made.
Prediction of Viral Filtration (VF) Performance of MAbs via Biophysical Analysis (William Rayfield, David Roush, Jason Cheung, Shehab Barakat, and Nihal Tugcu; Merck Research Laboratories)
Minimization of VF area by increasing flux as measured by liters per square meter per hour (LMH) and filter loading during development studies is critical to achieving cost targets. From a manufacturing perspective, “poor” virus filtration flux (<25 LMH) can greatly affect process cycle time as well as the target filter loading. Filter flux profiles are conserved between scales, with viral filtration scale-down models 103–104 times smaller than production scale. However, the scale-down model still requires ∼100–500 mL of feed material per run, often in excess of 1 g of MAbs. Analytical screening prior to process development can greatly reduce the amount of material needed for these studies.
The drivers for the research presented in this paper are (1) to identify effective analytical tools to aid the development of virus filtration while minimizing material needs and, (2) to establish a guidance of conditions based on application of these analytical tools to MAb programs. We present a case study where biophysical tools—high-performance size exclusion chromatography (HP-SEC), dynamic light scattering (DLS), and absolute size exclusion chromatography (ASEC)—were applied to a specific MAb program to illustrate how changes in feed composition (MAb concentration, pH, NaCl concentration, buffer salt type) can change biophysical properties of the feed that correlate with VF performance.
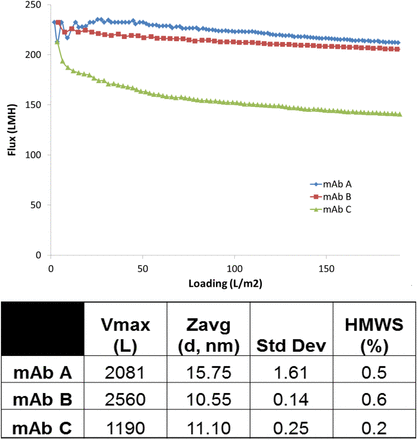
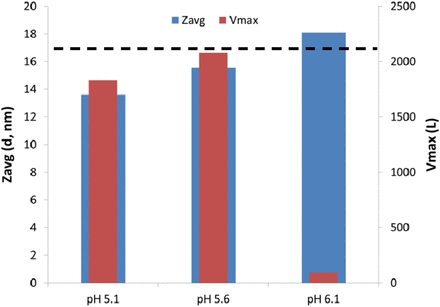
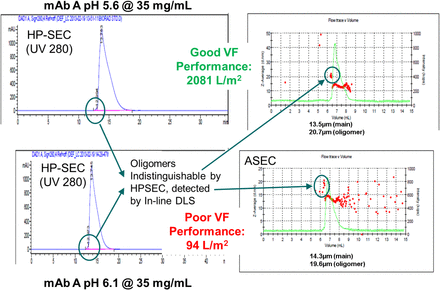
Many factors can affect virus filtration flux, including feed conditions (pH, conductivity, buffer salt type, etc.) and impurities [high molecular weight (HMW) species, subvisible particles, etc.]. There are instances when a MAb feed stream with relatively low HMW (≤1.0%) can still have poor performance, defined as reduced filter flux and loading as shown in Figures 11 and 12. For this reason, two methods have been used to highlight potential problem areas: DLS and in-line DLS (ASEC). DLS has shown promise in identifying problem areas in filter flux development optimization by allowing for the calculation of Zavg (mean hydrodynamic diameter) of a relatively clean feed stream. ASEC has been identified to be a method of evaluation as it connects HP-SEC with in-line DLS detection, which can provide a size (Zavg) to each HMW species peak (9) as shown in Figure 13. ASEC analysis suggests that the size of the dimer changes as a function of pH, for example, from 19.6 nm at pH 6.1 vs 20.7 nm at pH 5.6 for MAb A. One potential explanation for the difference in fouling behavior is that the smaller dimer at pH 6.1 allows for a deeper penetration into the asymmetric PES structure of Viresolve Pro, creating higher hydraulic resistance at the same operating pressure. It has been observed elsewhere that these oligomers can foul or constrict the filter pores to negatively affect filter flux (10). Significant differences in MAb Zavg (via DLS) affected fouling and loading on Viresolve Pro. However, despite the difference in Zavg between the early-stage and late-stage process (18.2 nm vs 13.5 nm) for MAb A, it did not affect LRV (<1 log) of Xenotropic murine leukemia virus–related virus (XMuLV) or MMV.
Impact of HMW species on VF performance (three MAbs).
Filtration performance of MAb A related to robustness screen. Filtration performed at 14 psi on Viresolve Pro 3.1 cm2 filter. Dotted line at 17 nm represents suggested cutoff for poor performance.
ASEC analysis of MAb A feed.
Analyses of feed attributes can then be applied to a decision tree to guide the recommendation of a VF filter and operating conditions for use in process development. Typically <10 mL of feed is needed to identify “good” acceptable virus filtration feed conditions, that is, robust filtrate flux of ≥30 LMH and ≥200 L/m2 on Planova 20N and ≥100 LMH and ≥500 L/m2 on Viresolve Pro.
Mechanistic Failure Mode Resolution and Comparison of Parvovirus-Retentive Filters (Daniel LaCasse, Pfizer; and Kurt Brorson, Center for Drug Evaluation and Research, U.S. Food and Drug Administration)
Virus-retentive filters are a key safety measure used in the production of biotherapeutic products. These filters are believed to function based on a purely size-based particle removal mechanism using mechanical sieving and retention of particles based on their hydrodynamic size. Recent observations have indicated changes in viral particle retention with changes in process pressures and flow interruptions. A mechanistic investigation was performed using several filter types to help identify a potential mechanism leading to reduced particle retention. The effect of permeate flow rate, or permeate driving force, was analyzed for its impact on particle retention in three commercially available small virus–retentive filters.
Lower virus filter transmembrane pressures (<70% recommended by filter manufacturer) and/or significant flow interruptions (>3 min) using typical low viscosity (<1.5 cP) process fluids led to a reduction in small virus and small bacteriophage removal across two of the filter types, while the third filter type exhibited little to no observed difference in retention. The results from this mechanistic investigation suggest that particle diffusion under reduced convective flow may lead to reduced particle retention in certain small virus–retentive filter types. Our finding that different small virus–retentive filter types appear to exhibit discernible differences in retentive performance across the pressure and/or flow regime argues that each filter type requires individualized process understanding and control.
Impact of Pressure and Pausing on Virus-Retentive Riltration (John Mattila; Regeneron)
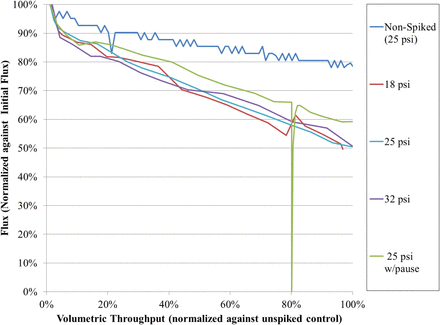
Regeneron assessed the performance of a 20 nm, dual-layer, polyethersulfone normal flow virus-retentive filter under varied flow conditions. The study was designed to identify worst-case operating conditions for use in MAb virus removal validation studies using a model immunoglobulin G (IgG4) process stream following publication of a pressure release failure mode (11). Tests were conducted using a 0.1% (v/v) spike of MMV to limit alteration of the feed stream. The dual-layer filter was tested at 18, 25, and 32 psi inlet pressure, while a final test evaluated virus breakthrough following a 1 h pressure release at 75% volumetric passage. The results of these experiments are shown in Figure 14.
Virus-retentive filtration pressure-flow profile. A model IgG4 spiked with 0.1% (v/v) MVM was filtered through a 20 nm dual-layer polyethersulfone filter at 18, 25, or 32 psi. Flux decay with spiked feed material is approximately 50% compared to 20% in a non-spiked control.
The investigation failed to identify a worst-case condition in the evaluated range because parvovirus infectivity was reduced to non-detectable levels in all cases. Furthermore, effective clearance exceeding 4.0 LRF was demonstrated for each pressure condition as shown in Table II. The results support published literature indicating sensitivity to flow conditions is filter brand–specific, and this 20 nm, dual-layer, polyethersulfone virus-retentive filter has not demonstrated virus passage following pressure release.
MVM Log10 Reduction Factor Achieved at Varied Pressure Conditions
Summary
In recent years, implementation of virus filters designed for parvovirus removal in the downstream process has become an industry standard and regulatory expectation for biologics manufactured in mammalian cell cultures. During the VF session, new approaches for small virus–retentive filter process development and viral clearance validation, as well as investigation of process parameters influencing parvovirus breakthrough, were presented.
Virus Filtration Process Development and Viral Clearance Validation Approaches
To support multiple virus filtration cycles within one harvest lot, Amgen proposed an alternative bracketed validation approach where the virus is spiked in the first and last cycles in the small-scale validation study and clearance is determined by the average clearance of the two cycles. Baxter BioScience presented an advanced, automated, down-scale model that allows MMV removal study of a high-volume throughput process (20,000 L/m2) with processing time up to 1 month and incorporating multiple pressure interruptions of various pause durations.
Due to their small pore size, small virus–retentive filters are prone to filter fouling. Merck presented a case study where DLS and in-line DLS (ASEC) were used to analyze the size of monomers and oligomers of a MAb feed stream at different pH. The data showed that the size variation correlates with the fouling behavior of the MAb, while viral clearance for both XMuLV and MMV are not affected. These tools are potentially useful to quickly identify optimal MAb feed conditions to develop a process with desired flux and volume or mass throughput. Biogen Idec reported using high-purity virus stock and improved feed material handling procedure to achieve high-volume throughput, effective virus removal, and significant cost savings.
Investigation of Process Parameters Affecting Parvovirus Breakthrough
Several parameters were found to have an impact on parvovirus breakthrough. Low pressure, sudden pressure decrease, and high load (g/m2) were shown to lead to PPV breakthrough, but protein concentration was not found to have any impact (Janssen). Eli Lilly reported that in addition to low pressure and DPRP, low pH and high conductivity also affect PPV retention. DPRP for a buffer flush step can be avoided through engineering solutions to eliminate it as a risk factor for parvovirus breakthrough.
GlaxoSmithKline presented PPV removal by the Planova 20N filter using five MAbs. The filter exhibited little fouling; however, PPV LRVs were dependent on total virus load. When the filter was challenged with an increasing total virus load from 105 to 108 PFU per small-scale filter, the PPV removal increased from 3 to 6 LRV. When the total PPV load was further increased from 108 to 1010 PFU, PPV clearance decreased from 6 to 3 LRV due to increase of PPV breakthrough. It is interesting to note that when more viruses are loaded, more are retained by the filter (approximately 1010 vs 108 total PPV PFU) despite the higher level of breakthrough resulting in lower LRV. It thus seems to be justifiable to optimize the virus spike to achieve the highest LRV claim knowing that higher spike will lead to more virus particles being removed despite a lower LRV.
Pfizer analyzed small virus and bacteriophage removal in three commercially available filters. It was found that particle diffusion under low pressure/flow might cause virus breakthrough in certain virus filter brands. This finding was consistent with data shown by Regeneron that MMV removal was not affected by low pressure and pressure release in the filter brand tested. These data indicate that sensitivity to parameters such as low pressure is filter brand–specific.
Next Steps
While significant efforts have been made in recent years, there are still knowledge gaps to fully understand the mechanism of virus breakthrough by small virus–retentive filters (3). Current filters on the market have improved compared to the first-generation filters; however, they are still not ideal with sensitivity to either parvovirus breakthrough or fouling, which requires longer development timeline. Additional studies are needed in order to fill these gaps and better understand the factors involved. A better understanding of parvovirus passage is important in order to design the most meaningful viral validation studies, define critical process parameters (CPPs) and appropriate controls in manufacturing, and develop the next generation of small virus–retentive filters that reliably provide high throughput capacity and robust virus retention capabilities. It has been reported recently that parvovirus breakthrough and filter performance can be influenced by multiple factors such as pH, quality of virus preparations, products, and pressure release (11–15). Combined with the fact that parvovirus filters differ in their chemistry and configuration, it is unrealistic to expect that CPPs identified from a given study with a particular brand of parvovirus filter will be universally applicable.
- © PDA, Inc. 2015