Abstract
Ethanol/water mixtures are frequently used as simulating solvents in extractables studies. However, the basis for determining and justifying the right ethanol proportion in a simulating solvent for a particular drug product or solution has not been previously established.
A solvent strength model has been developed in this study, based on the correlation between the levels of a model compound, di-(2-ethylhexyl) phthalate (DEHP), extracted from a reference source material, plasticized poly-(vinyl chloride) (PVC) resin, and the proportion of ethanol in ethanol/water extractions solvents. This model was established by experimentally investigating DEHP leaching and takes the form:
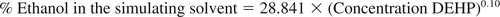 If the level of DEHP extracted from the standard source PVC resin by a drug product is measured, then the level can be input into the above equation and the proper ethanol content of the appropriate simulating solvent can be determined.
If the level of DEHP extracted from the standard source PVC resin by a drug product is measured, then the level can be input into the above equation and the proper ethanol content of the appropriate simulating solvent can be determined.
The model has been applied to certain drug products and additives used in drug products, and the proper ethanol/simulating solvents for these products have been established. Additionally, the leaching behavior revealed in this study has been established to be consistent with previously published research and a mechanism for the observed behavior has been proposed.
LAY ABSTRACT: Although ethanol/water mixtures are frequently used as simulating solvents in extractables studies, it is difficult to establish and justify what the right proportion of ethanol is for a particular drug product. A solvent strength model has been developed based on the leaching behavior of a model compound, di-(2-ethylhexyl) phthalate (DEHP), from a reference source material, plasticized poly-(vinyl chloride) (PVC). By measuring the level of DEHP leached into a drug product from the reference source material, one can use the model to calculate the correct ethanol proportion in the simulating solvent.
Using this approach, ethanol/water proportions have been obtained for certain drug products. Additionally, the leaching profiles for DEHP obtained in this study were noted to be consistent with such profiles for other extractables from the PVC reference source material and with other investigations of ethanol/water as model stimulants.
- © PDA, Inc. 2015
PDA members receive access to all articles published in the current year and previous volume year. Institutional subscribers received access to all content. Log in below to receive access to this article if you are either of these.
If you are neither or you are a PDA member trying to access an article outside of your membership license, then you must purchase access to this article (below). If you do not have a username or password for JPST, you will be required to create an account prior to purchasing.
Full issue PDFs are for PDA members only.
Note to pda.org users
The PDA and PDA bookstore websites (www.pda.org and www.pda.org/bookstore) are separate websites from the PDA JPST website. When you first join PDA, your initial UserID and Password are sent to HighWirePress to create your PDA JPST account. Subsequent UserrID and Password changes required at the PDA websites will not pass on to PDA JPST and vice versa. If you forget your PDA JPST UserID and/or Password, you can request help to retrieve UserID and reset Password below.






