Abstract
This study is to investigate the effect of headspace air pressure in pre-filled syringes on liquid leak (dripping) from the syringe needle upon needle shield removal. Drip tests to measure drip quantity were performed on syringes manually filled with 0.5 or 1.0 mL of various aqueous solutions. Parameters assessed included temperature (filling and test), bulk storage conditions (tank pressure and the type of the pressurized gas), solution composition (pure water, 0.9% sodium chloride, and a monoclonal antibody formulation), and testing procedures. A headspace pressure analyzer was used to verify the drip test method. Results suggested that leakage is indeed caused by headspace pressure increase, and the temperature effect (ideal gas expansion) is a major, but not the only, factor. The dissolved gases in the liquid bulk prior to or during filling may contribute to leakage, as these gases could be released into the headspace due to solubility changes (in response to test temperature and pressure conditions) and cause pressure increase. Needle shield removal procedures were found to cause dripping, but liquid composition played little role. Overall, paying attention to the processing history (pressure and temperature) of the liquid bulk is the key to minimize leakage. The headspace pressure could be reduced by decreasing liquid bulk storage pressure, filling at a higher temperature, or employing lower solubility gas (e.g., helium) for bulk transfer and storage. Leakage could also be mitigated by simply holding the syringe needle pointing upward during needle shield removal.
LAY ABSTRACT: Substantial advances in pre-filled syringe technology development, particularly in syringe filling accuracy, have been made. However, there are factors, as subtle as how the needle shield (or tip cap) is removed, that may affect dosing accuracy. We recently found that upon removal of the tip cap from a syringe held vertically with needle pointed downwards, a small amount of solution, up to 3–4% of the 1 mL filled volume or higher for filled volume of <1 mL, leaked out from the needle. This paper identified the root causes of this problem and offered solutions from the perspectives of the syringe fill process and the end user procedure. The readers will benefit from this paper by understanding how each process step prior to and during syringe filling may affect delivery performance of the pre-filled syringe device.
Introduction
Large molecules like monoclonal antibodies, proteins, and peptides need to be delivered via the parenteral route. Pre-filled syringes are now the primary container of choice for most parenteral drug delivery systems mainly for reasons that they are safe and user friendly (1). In recent years substantial advances in pre-filled syringe technology development have been made, particularly in syringe filling accuracy (2). However, there are factors, as subtle as how the needle shield (or tip cap) is removed, that may affect dosing accuracy. We recently found that upon removal of the tip cap from a syringe held vertically with needle pointed downwards, a small amount of solution (up to 3–4% of the 1 mL filled volume) leaked out from the needle. Although holding the syringe tip downwards during needle shield removal is not recommended, this remains a significant issue affecting the overall delivery dose, especially with small fill volumes.
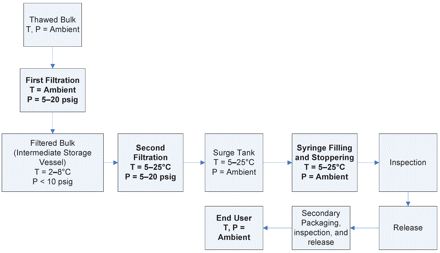
The same observation was previously reported by Kinney and co-workers who attributed such leakage to bubble expansion as the result of small vacuum created during tip cap removal (3). We agree that the root cause of leakage is the air bubble (or headspace) and that the vacuum created by tip cap removal can cause bubble expansion, thereby resulting in liquid dripping. This phenomenon, however, may be more complex. Actually, any factors capable of causing air bubble expansion may be able to induce leakage. Certainly, temperature increase (e.g., from refrigeration to an ambient environment) could cause bubble expansion simply following the Ideal Gas Law (described below). Yet, this study would go far beyond to investigate upstream process effects. Prior to filling, thawed liquid bulks are filtered (under a trans-membrane pressure) and stored in a tank pressurized under nitrogen blank for some time before second filtration (under a trans-membrane pressure) (Figure 1). Certainly, all these steps up to liquid fill can be performed under a controlled temperature and/or pressure. Thus, this study investigates whether and how the temperature and pressure history of the liquid may eventually affect headspace expansion/pressure. Furthermore, procedures of needle shield removal were assessed to better understand the leakage phenomenon. Three aqueous solutions, pure water, 0.9% NaCl, and an anti-IgE monoclonal antibody formulation, were tested in this study to understand the effect of formulation on leakage.
Process flow diagram of syringe filling.
Materials and Methods
All experiments in this study employed 1.0 mL long 26G½ inch staked needle syringes filled with 1.0 mL or 0.5 mL volume of pure water, 0.9% NaCl aqueous solution, or an anti-IgE monoclonal antibody aqueous solution. All solutions were 0.22 μm–filtered and stored at 2–8 °C under a pre-determined pressure. All pre-filled syringes were manufactured and manually filled in the laboratory. Two different lots of syringe barrel (pre-assembled with needle shields) were used to demonstrate reproducibility. Samples were analyzed by measuring gross weight change using an analytical balance before and after removal of the rigid needle shield. Equipment and materials used in this study are tabulated in Table I.
Equipment and Materials Used in the Study
The following describes the processing and test procedures for pre-filled syringes manufactured for this study.
Components Sterilization
Empty syringes with 26G½ inch staked needle (previously washed and siliconized by an external contract manufacturer), W4023/FLT stoppers, and stopper insertion tools were autoclaved using a conservative ramp cycle (ramps at 1 psi/min, 30 min exposure at 121.1 °C, and 30 min drying at 2 psia) during pressurization and evacuation to minimize the chance of needle shield movement during the sterilizing process. After the cycle was complete, each syringe was visually inspected for loose needle shield.
Liquid Bulk Storage under Pressurization
A 5 L stainless steel pressure tank was assembled with a calibrated pressure gauge, a valve connected with a vent filter, and a pressure regulator connected to a gas source. Each filtered solution was individually loaded into the pressure tank and pressurized to a desired pressure (0, 10, or 20 psig) and held at 2–8 °C (cold room) for 24 h. The pressure gauge was checked periodically to ensure constant pressure was maintained.
Manual Syringe Filling and Stopper Insertion
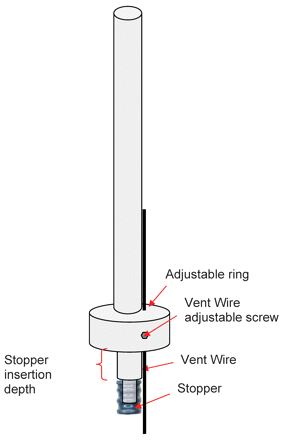
The pressure tank was depressurized in the cold room to 0 psig by closing the gas source and opening the vent filter valve. A liquid volume of 1.0 mL or 0.5 mL was withdrawn from the pressure tank using a repeater pipette and filled into 1 mL long syringes. W4023/FLT plunger stoppers were manually inserted using a Becton Dickinson (BD) stopper insertion tool as illustrated in Appendix I. The depth of the stopper (measured from the back of the syringe flange to the back of the stopper) was positioned at 8 mm for 1 mL syringes and 24 mm for 0.5 mL syringes. This resulted in headspace height of approximately 8 mm for both configurations.
Although the majority of the syringes were filled in the cold room at 2–8 °C, some were filled at higher temperatures (15 °C and 21.5 °C) using a circulation bath to control the liquid temperature. In that case a digital thermometer was submerged directly in the solution to monitor the actual fill temperature. All samples were stored at 2–8 °C after filling for at least 24 h to allow the headspace pressure to equilibrate.
Drip/Leakage Test
Unless otherwise specified, all filled syringes were equilibrated to ambient temperature (measured and recorded) for at least 30 min before testing. The initial syringe weight, Winitial, was measured. Syringes were held vertically with the needle pointing downward—except for samples tested for different rigid needle shield (RNS) removal methods—and the needle shield was then removed to allow for solution dripping from the tip of the needle on water-absorbent material (e.g., paper tissue). When the needle stopped dripping, the syringe and the needle shield were weighed together to obtain the leakage amount by taking the difference in weight before and after the drip. Finally, to determine the liquid extractable volume in each syringe, a plunger rod was inserted to eject all liquid content from the syringe. Empty syringe weight (including the needle shield), Wempty, was then determined, and the gross weight of the dripped solution could be calculated accordingly.
Headspace Pressure Measurement
An optical-based, non-destructive gas analyzer (Model FMS-1400P, Lighthouse Instruments) was employed to determine the headspace pressure inside sealed prefilled syringes. Briefly, light from a near-infrared laser is tuned to match an internal absorption frequency of the water molecule and passes through a container in the headspace above the product. The amount of laser light absorbed is proportional to the water vapor concentration in the headspace, while the width of the absorption signal is related to the headspace pressure (4).
Pressure and Temperature of Gas Bubble Calculation (Ideal Gas Law)
Based on the Ideal Gas Law (eq 1), when syringes were filled at 5 °C (T1) under ambient pressure (P1) and then relocated to an ambient environment at use (P2 and T2) for drip test, the air bubble would experience volume and/or pressure increase. After the needle shield was removed, the open system allowed the volume of the air bubble to expand until the bubble pressure equilibrated with the ambient pressure (P1 = P2). Bubble expansion (V2) displaced some solution as leakage and the expanded volume (V2 − V1) could be theoretically calculated by eq 2.

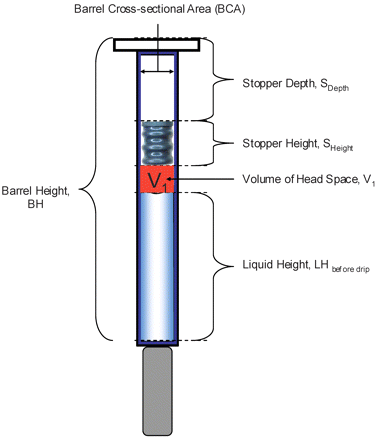
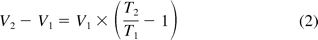 where T1 and T2 are known values, initial and final temperatures, respectively; and V1 is a constant that can be calculated from known syringe physical parameters (eq 3 and Figure 2).
where T1 and T2 are known values, initial and final temperatures, respectively; and V1 is a constant that can be calculated from known syringe physical parameters (eq 3 and Figure 2).
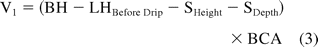 where BH is the barrel height, LHBefore Drip is the liquid height before removal of the needle shield, SHeight is the stopper total height, SDepth is the stopper position measured from the back of the syringe flange to the back of the stopper, and BCA is the barrel cross-sectional area.
where BH is the barrel height, LHBefore Drip is the liquid height before removal of the needle shield, SHeight is the stopper total height, SDepth is the stopper position measured from the back of the syringe flange to the back of the stopper, and BCA is the barrel cross-sectional area.
Graphic representation of syringe and its physical dimensional parameters used in eq 3.
Conversion of Drip Test Results into Pressure Units (Torr)
Before and after the drip test, the headspace conditions in the filled syringe are (T1, P1, V1) and (T2, P2, V2), respectively. P1 represents the internal pressure of the syringe headspace before the drip test, and P2 represents ambient pressure or is 1 atm.; V1 and V2 can be calculated using eqs 3 and 4 and physical dimensions (Table II):
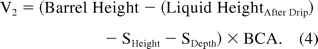 Once V1 and V2 are calculated, assuming T1 = T2, the Ideal Gas Law (eq 1) can be used to derive eq 5 and solve for P1:
Once V1 and V2 are calculated, assuming T1 = T2, the Ideal Gas Law (eq 1) can be used to derive eq 5 and solve for P1:

Physical Dimensions of Syringe, Components, and Liquid Fill
Results and Discussion
Effect of Pressure and Temperature during Bulk Storage and Filling
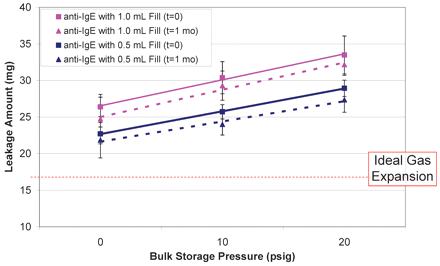
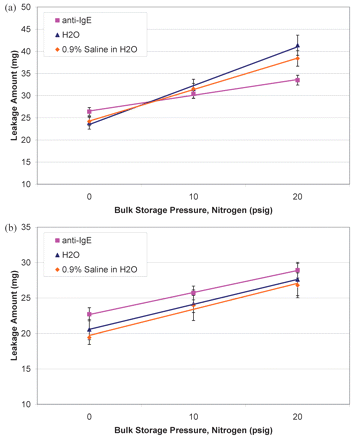
Leakage was first tested on syringes manually filled with the anti-IgE antibody formulation that had been stored under N2 blank at three different pressures: 0, 10, and 20 psig. The results are summarized in Figure 3. The ideal gas expansion of inert nitrogen, calculated using eq 2 with the temperature change from filling at 5 °C to testing at 21.5 °C, was 17 mg (the horizontal line in Figure 3) based on density of 1.06 g/L. Both 0.5 mL and 1.0 mL syringes leaked more than the dripping contributed by ideal gas expansion. In addition, the amount of leakage increased with increasing storage pressure, approximately 0.3 mg/psig. The 1.0 mL syringes appeared to expel more liquid, 25–33 mg (6–8 drips or 2.4–2.8% of the overall dose) than the 0.5 mL syringes, 23–29 mg (5–7 drips or 4.6–5.0% of the overall dose) over the whole pressure range. These observations could be attributed to increased gas solubility. When the storage pressure increased, it dissolved more nitrogen molecules into the solution. Some of dissolved N2 would escape to the headspace created during filling under ambient pressure, thereby enhancing the headspace pressure. Because 1.0 mL filled syringes contained twice the liquid volume, more dissolved gas diffused from the liquid to the gaseous phase, which resulted in slightly higher headspace pressure than the 0.5 mL filled counterpart.
Effect of liquid bulk storage pressure (0, 10, and 20 psig) on leakage from syringes filled with 1-mL or 0.5-mL of anti-IgE antibody liquid formulation. Syringes tested at t=0 and t=1month are represented by square and triangle symbols, respectively. The two fill volumes of 1.0 mL and 0.5 mL are illustrated by pink and blue color, respectively.
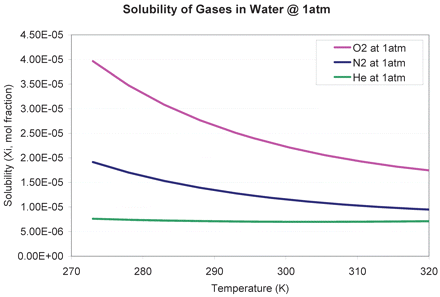
It is worth noting that the storage pressure effect could not explain the 0 psig storage condition where the leakage was still higher than that predicted by ideal gas expansion. Again, it is a gas solubility phenomenon and could be interpreted by the temperature-solubility relationship. Gas solubility in water generally decreases with increasing temperature. Nitrogen solubility can be predicted as a function of temperature as illustrated in Appendix II (5–8), showing that nitrogen solubility decreases from 1.7 × 10−5 (mole fraction) at 5 °C (278 °K) to 1.25 × 10−5 (mole fraction) at 21.5 °C (294.5 °K). Thus, during the leakage test at ambient temperature, some nitrogen and oxygen molecules originally dissolved in the liquid during filling at 2–8 °C became insoluble and would diffuse to the headspace, which caused higher pressure and additional leakage. Note that oxygen is even more soluble than nitrogen (Appendix II).
Some pre-filled syringes were stored at 2–8 °C for 1 month as part of a stability study and tested for leakage. The results (Figure 3) were similar to the T = 0 condition, suggesting the additional storage time has little effect on leakage, which was primarily determined by the temperature and pressure of liquid storage, filling, and leakage test.
Effects of Gas Species
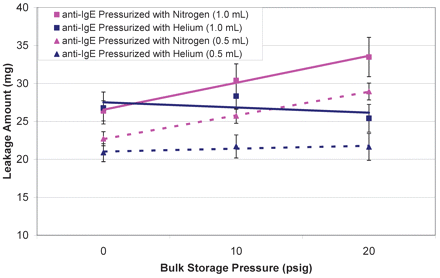
Because a soluble gas like N2 contributed to headspace pressure increase, a less soluble gas might alleviate such an effect. As shown in Appendix II, helium is less soluble than N2, ∼0.7 × 10−5 mole fraction, and is independent of temperature changes. Drip tests were performed on syringes filled with liquid bulks previously stored under helium at three different pressures: 0, 10, and 20 psig. It is apparent that helium caused less leakage than N2, and both 0.5 mL and 1.0 mL filled syringes showed little changes in leakage amount with pressure (Figure 4). Again, the 0 psig result exceeds what is predicted by the Ideal Gas Law and can be attributed to soluble gases (oxygen and nitrogen) that already dissolved in the liquid during storage and filling (2–8 °C). The dissolved gases could have diffused into the headspace during the leakage test at the higher ambient temperature.
Effect of gas species (nitrogen vs argon) on leakage from syringes filled with 1 mL or 0.5 mL of anti-IgE antibody liquid formulation. Syringes with fill volumes of 1.0 mL and 0.5 mL are represented by square and triangle symbols, respectively. The two inert gases, nitrogen and helium, are illustrated by pink and blue colors, respectively.
Effects of Liquid Composition
Liquid salinity may influence gas solubility; higher salinity in the solution may have a tendency to reduce gas solubility (6). The anti-IgE antibody formulation contains arginine HCl, which makes the formulation slightly higher in salinity than water. In addition, a 0.9% NaCl solution was evaluated. The leakage results on these three liquids showed no clear trend of salinity effects (Figure 5a for 1.0 mL filled syringes and Figure 5b for 0.5 mL filled syringes) when all three storage pressures (0, 10, and 20 psig) were compared. This leakage phenomenon appeared to be insensitive to formulation changes given the test conditions.
Effect of liquid bulk composition (anti-IgE antibody formulation [pink square], pure water [blue triangle], and 0.9% NaCl aqueous solution [orange diamond]) on leakage from syringes filled with 1.0 mL liquid (a) or 0.5 mL liquid (b).
Effect of Liquid Fill and Drip Test Temperature
Temperature is certainly one of the most important factors influencing the amount of leakage, particularly the temperature during liquid fill and drip test. The former represents the final equilibration condition that the liquid experiences before being sealed in the syringe. Assuming the liquid is filled and tested under the same ambient pressure, higher test temperature/lower fill temperature would result in bubble expansion and liquid leakage, but lower test temperature/higher fill temperature would cause bubble contraction and no liquid leakage. Theoretically there should be little or no leakage if fill temperature and test temperature are identical. This temperature effect takes both ideal gas expansion/contraction and gas solubility into consideration.
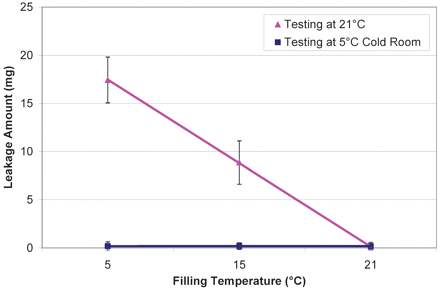
Syringes were manually filled with 1 mL of pure water at three temperatures, 5, 15, and 21.5 °C and tested for leakage at 5 and 21.5 °C at ambient pressure (0 psig). The results (Figure 6) confirmed that leakage can be minimized or completely avoided if the liquid fill temperature is equal to or higher than the drip test temperature, particularly filling at 21.5 °C resulting in almost no leakage. Thus, performing liquid fill at higher temperatures (if protein stability allows) is one of the most effective approaches to mitigate leakage when the needle shield is removed.
Effect of filling temperature (5° C, 15° C, and 21.5° C) and drip test temperature (5 °C [blue square] and 21.5 °C [pink triangle]) on leakage from syringes filled with 1.0 mL of pure water.
Effect of Needle Shield Removal Methods
Kinney and co-workers reported that needle shield removal creates vacuum at the tip of the needle which promotes leakage (3). This vacuum effect is probably inevitable. Therefore, this part of the study was to evaluate some needle shield removal procedures and identify the one having the least effect on leakage.
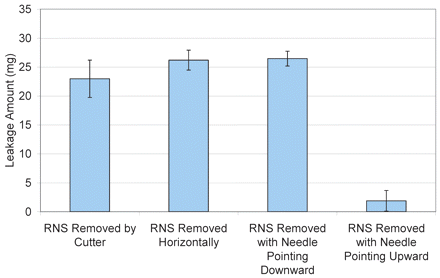
Syringes manually filled with 1.0 mL of pure water (under 0 psig bulk storage pressure) were tested by holding the syringe either vertically (needle tip pointing up and down) or horizontally and by carefully cutting the needle shield open (lengthwise into halves) to avoid or minimize any vacuum that may be created from pulling on the rubber part of the needle shield. As shown in Figure 7, the vertical (needle tip pointing downwards) and horizontal removal methods showed no observable differences and the amount of leakage remained high (25–27 mg). The cutting removal methods had a mild effect in reducing leakage (20–25 mg), suggesting leakage contributed by vacuum from removing the needle shield is minor. Vertically removing the needle shield with needle pointing upwards was most effective and caused almost no leakage. With the needle pointing upwards, the air bubble flowed to the top of the syringe, allowing air to vent for pressure reduction. This is the simplest solution to this leakage issue if users follow this recommendation.
Effect of rigid needle shield removal methods on leakage from syringes containing 1.0 mL of pure water filled at 5 °C and 0 psig.
Headspace Pressure Measurement
An optical gas analyzer (Model FMS-1400P, Lighthouse Instruments) was employed to directly measure headspace pressure in syringes filled with 1.0 mL of pure water that have been stored under a pressure of 0 psig or 20 psig prior to fill. In the meanwhile, the amount of leakage (in weight) measured by the drip test could be converted to pressure (Torr) using eqs 4 and 5 along with the physical dimensions in Table II. The results are summarized in Table III. The measured headspace pressure agreed well with what is derived from the drip test, suggesting that the drip test is a good tool for headspace pressure measurement and that headspace pressure increase is indeed the root cause of leakage.
Comparison of Headspace Pressure Measured Using an Optical Gas Analyzer and Derived from Drip Test (n = 5) in Syringes Filled with 1.0 mL of Pure Water
Conclusions
Liquid leakage from the pre-filled syringe after needle shield removal is a phenomenon universal to all aqueous liquids. Other than the vacuum created during needle shield removal, we demonstrated that the root cause of leakage is an increase in headspace pressure dictated by multiple parameters, mainly the temperature and pressure history of the liquid bulk during the filling manufacturing process. Headspace pressure increase was primarily caused by ideal gas expansion and soluble gas re-equilibration between the liquid and the gas phases. We determined that performing liquid fill at a temperature close to a standard ambient temperature (∼20 °C) or removing the needle shield while pointing the needle upward is most effective in minimizing leakage. Lowering liquid bulk storage/filtration pressure or using a low solubility gas (e.g., helium) for storage/filtration is also useful in alleviating headspace pressure/leakage.
Conflict of Interest Declaration
The author(s) declare that they have no competing interests.
Appendix I. Stopper Insertion Tool (not drawn to scale)
Appendix II. Solubility Profile of Different Gases in Water at 1 atm
According to Reference 7, the mole fraction solubility X1 of the gas can be derived from the smoothing equation at each temperature:
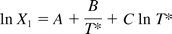
where
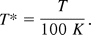
Plugging in all the constants from the table below, a solubility profile can be plotted from 273K to 328K.
- © PDA, Inc. 2011