Abstract
A 36 month leachable study on water for injection in direct contact within a polymer-based prefillable syringe consisting of a cyclo olefin polymer barrel, a chlorinated isoprene isobutene rubber plunger stopper, a polymer label attached on the barrel, and a secondary packaging was conducted at 25 ± 2 °C and 60 ± 5% relative humidity. Through the various comparison studies, no difference in the leachable amounts was observed between this polymer-based prefilled syringe and a glass bottle as a blank sample reference by 36 months. No influence on the leachables study outcome was noted from the printed label and/or label adhesive or from the secondary packaging. In an additional study, no acrylic acid used as the label adhesive leachable was detected by an extended storage for 45 months at 25 ± 2 °C and 60 ± 5% relative humidity as a worst case. To obtain more details, a comparison extractable study was conducted between a cyclo olefin polymer barrel and a glass barrel. In addition, chlorinated isoprene isobutene rubber and bromo isoprene isobutene rubber were compared. As a result, no remarkable difference was found in the organic extractables for syringe barrels. On the other hand, in the case of element extractable analysis, the values for the cyclo olefin polymer barrel were lower than that for the glass barrel. For the plunger stoppers, the chlorinated isoprene isobutene rubber applied in this study was showing a lower extractable profile as compared to the bromo isoprene isobutene rubber, both for organic and element extractables. In conclusion, the proposed polymer-based prefillable syringe system has great potential and represents a novel alternative that can achieve very low level extractable profiles and can bring additional value to the highly sensitive biotech drug market.
LAY ABSTRACT: A 36 month leachable study on water for injection in direct contact within a cyclo olefin polymer barrel and chlorinated isoprene isobutene rubber plunger stopper that has a polymer label attached to the barrel and is wrapped into a secondary packaging was conducted at 25 °C and 60% relative humidity. Through the various comparison studies, no difference in the leachable amounts was observed between polymer-based prefilled syringes and a glass bottle as a blank sample reference by 36 months. No influences on the leachables study outcome were noted from the secondary packaging. To obtain more details, a comparison extractable study was conducted between the cyclo olefin polymer and the glass barrel. In addition, chlorinated isoprene isobutene rubber and bromo isoprene isobutene rubber plunger stoppers were compared as well. As a result, no remarkable difference was found in the organic extractables for barrels. As for element extractable analysis, the values for the cyclo olefin polymer barrel were lower than that for the glass barrel. For the plunger stoppers, the chlorinated isoprene isobutene rubber applied in this study was showing a lower extractable profile as compared to the bromo isoprene isobutene rubber, both for organic and element extractables. In conclusion, the proposed polymer-based prefillable syringe system has great potential and represents a novel alternative that can achieve very low level extractable profiles and can bring additional value to the highly sensitive biotech drug market.
- Cyclo olefin polymer
- Chlorinated isoprene isobutene rubber
- Butyl rubber
- Label
- Label printing
- Adhesive
- Secondary packaging
- Extractable
- Leachable
- Stability
- Prefilled
- Prefillable syringe
1. Introduction
Increase in the use of prefilled syringes (PFSs) is motivated by many advantages over traditional ampoules and vials. These advantages include quick and accurate dosing, reduction in the risk of biological contamination, greater convenience with ease of use, and reduction of overfill volume as compared to vials and ampoules and thus reducing drug wastage. The increase in demand in the use of PFSs is driven by the growing availability of biological drugs in recent years.
Containers for PFSs have to meet various functional requirements; for instance, container closure integrity, shock resistance, plunger break-loose and gliding forces, waste disposal, and so on. PFSs consist of various components; materials commonly used are glass, polymers, elastomers components. Material selection for the components have to be made appropriately to ensure meeting the requirements for the intended use.
Among them, glass PFSs are used extensively and have been substantive for the development of parenteral drugs. However, with the increased availability of biopharmaceuticals and the emergence of biosimilars, several issues related to the properties of glass material, such as protein aggregation and oxidation, are still unresolved (1⇓⇓⇓⇓⇓⇓⇓–9). Furthermore, considering the high production cost of biopharmaceuticals, product loss caused by container breakage during manufacturing, transportation, and use cannot be ignored.
Recently, polymer-based PFSs have been utilized as an alternative to glass PFS to overcome these issues with glass PFS. Polymer-basedPFSs offer the benefits of consistent and high-dimensional reproducibility and precise processing that allow product design to be flexible in developing customized syringes. There are several polymer materials available in the industry, including polypropylene (PP), cyclic olefin copolymer (COC), and cyclo olefin polymer (COP). Among those, COP is one of the advantageous materials used for a parenteral drug container due to, among other properties, its high transparency and high break resistance. For the materials of plunger stoppers and caps of PFS, several kinds of rubber and elastomers are commonly used. Chlorinated isoprene isobutene rubber (CIIR) and bromo isoprene isobutene rubber (BIIR) are typical examples available in the market today.
In order to apply biopharmaceuticals in a PFS format, compatibility with the drug container is one of the most important points to be investigated, as most of the biopharmaceuticals are physically and chemically sensitive. Especially, it is known that leachables, which migrate from the container, can denaturize the protein drug product, lower the drug efficacy, and may pose safety issues for the patient (10⇓⇓–13).
To assess and minimize the risks in the development process of biopharmaceuticals, information about the identity and amount of substances provided by extractables/leachables study gives us valuable insights. As a matter of fact, extractables/leachables from drug containers have been extensively discussed recently (14⇓⇓⇓–18) because it is crucial to confirm the extractable and leachable profile and toxicity concern to understand drug compatibility. However, most of the reports are related to glass PFSs. And the extractable and leachable profile of polymer-based PFSs has not been well discussed yet.
The purpose of this study is to confirm the extractable and leachable profile of a polymer-based PFS in conjunction with the selected CIIR elastomeric closures. Furthermore, extractable profiles of conventional glass PFSs are compared.
2. Materials and Methods
2.1. Materials
2.1.1. Materials for Leachable Study:
A PLAJEXTM syringe (5 mL luer lock syringe) and plunger stopper lubricated by silicone oil was provided by Terumo Co. (Tokyo, Japan), which is a polymer-basedPFS consisting of a COP barrel and a CIIR plunger stopper. Water for Injection (WFI) was European Pharmacopeia grade. All other chemicals and reagents were of analytical grade. As a reference, an inert glass bottle was used.
2.1.2. Materials for Extractable Study:
A 1 mL PLAJEXTM syringe consisting of the same materials as the sample used in the leachable study was provided by Terumo Co. (Tokyo, Japan). As a reference, a conventional glass barrel and BIIR plunger stopper were included in this study.
2.2. Sample Preparation
2.2.1. Leachable Study:
PLAJEXTM syringes were filled with 5 mL of WFI. After labeling with an acrylic-based self-adhesive glue on the filled syringes, the syringes were individually wrapped in the polymer-based blister pack as a secondary packaging (Table I). The sample syringes used for the leachable study were stored horizontally together with the respective blank solution (in inert glass bottles) in a monitored climate chamber for 36 months at 25 ± 2 °C and 60 ± 5% relative humidity. Intermediate samples were taken after storage times of 0, 1, 3, 6, 12, 24, and 36 months. For only the glue-related acrylic acid leachable study, the storage period was 45 months as a worst case.
Component Materials Used for the Leachable Study
2.2.2. Extractable Study:
About 11 g of each test sample (glass barrel, COP barrel, BIIR plunger stopper, and CIIR plunger stopper) was taken, and 110 mL of WFI was filled in a glass bottle and then treated at 121 °C for 1 h in an autoclave. One hundred mL of extracting solution and 100 mL of dichloromethane (DCM) were filled with the appropriate size of separatory funnel. This separatory funnel was shaken for 20 min; after that, the separated DCM layer was moved into the eggplant-shaped flask. This extraction was repeated one more time. Two hundred mL of the DCM layer (total amount of 100 mL of 1st and 2nd extraction DCM layers) was concentrated until its volume became about 2 mL using a rotary evaporator. Two mL of DCM solvent was separated from this concentrated layer using a measuring flask, and gas chromatography/mass spectrometry (GC/MS) analysis was performed. After GC/MS analysis, 2 mL of DCM solvent was diluted 5 times with methanol, and liquid chromatography/mass spectrometry (LC/MS) analysis was performed.
The glass barrel, CIIR plunger stopper, and BIIR plunger stopper were treated using the same methods as the COP barrel. In case of blank, only the WFI was treated using the same methods.
2.3. Analytical Method
2.3.1. Analytical Method for Leachable Study:
The analytical methods for the identification and quantification of leachables were carried out using a broad range of survey-type techniques and methods. These techniques are summarized in Table II.
Summary of Analytical Methods for Survey-Type Techniques
2.3.1.1. Preparation of Analytical Samples for GC/MS and LC/MS:
Two hundred mL of the test solutions were extracted three times, first without pH adaptation (pH 5.5–6.0), then at pH < 3 (1.5–2.0) and at pH > 12 (12.5) with 40 mL portions of DCM. The combined extracts were concentrated to 2 mL under a nitrogen flow (concentration factor 100) and 20 μL of an internal standard solution mix in methanol containing 990 mg/L of 2-fluorobiphenyl, and 206 mg/L of Tinuvin 327 were added. The spiked sample concentrates were divided over different 2 mL autosampler vials to be supplied for the different analytical methods.
2.3.1.2. Volatile Organic Compounds (VOC) by Headspace (HS)-GC/MS:
Thirteen mL of each test solution were transferred into separate 20 mL headspace vials containing 4.0 g of anhydrous Na2SO4. Twenty μL of an internal standard solution containing 39.8 mg/L of Toluene-d8 in methanol was added to obtain a final concentration of 61.23 μg/L of internal standard. The headspace vials were tightly capped and consecutively heated to 75 °C for 20 min. Subsequently, each headspace content was transferred to the GC/MS for injection and analysis (GC/MS: Agilent 7683 Auto sampler with Agilent 6890N Network GC System, Agilent 5973 Inert Mass Spectrometric Detector, Column: HP-5MS 30 m × 0.25 mm × 0.25 μm).
The concentration of detected volatile compounds can be estimated by a semi-quantitative internal calibration method. The relative analytical response of a reported compound is compared to the response of the internal standard (Toluene-d8, added to the samples at a fixed concentration). The concentrations in the original samples are calculated according to the following formula:
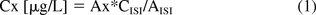 where Cx is the concentration of the target compound in the original sample, Ax is the measured peak area of the target compound in the sample extract, CISI is the spiked concentration of the internal standard for injection (ISI), and AISI is the measured peak area of ISI.
where Cx is the concentration of the target compound in the original sample, Ax is the measured peak area of the target compound in the sample extract, CISI is the spiked concentration of the internal standard for injection (ISI), and AISI is the measured peak area of ISI.
2.3.1.3. Semi-Volatile Organic Compounds (SVOC) by GC/MS:
2.3.1.4. Non-Volatile Organic Compounds (NVOC) by LC/MS:
LC/MS analysis was performed on the spiked final DCM extracts. Due to different ionization preferences of possible target compounds, the analysis was performed in two separate analytical runs, applying either positive (APCI+) or negative (APCI–) ionization mode under the LC conditions given below.
To quantify detected compounds, the chromatogram of a selected ion is extracted with total ion current chromatography (TIC) and traced in an extracted ion chromatogram (EIC). The concentration of the detected peaks can be determined using Tinuvin 327 as internal standard. The peak area of the extracted ion is compared to the peak area of the characteristic ion of ISI, which was spiked to the sample at a known concentration.
A compound-specific relative response factor (RRF) was determined from a calibration standard of 5 mg/L (50 mg/L for fatty acids). The RRF was used for the more accurate quantification of identified target compounds (IC). For other compounds, the RRF is assumed to be 1.
The concentrations in the original samples are calculated according to the following formula:
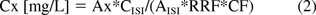 where Cx is the concentration of the target compound in the original sample, Ax is the measured peak area of the target compound in the sample extract, CISI is the spiked concentration of ISI, AISI is the measured peak area of ISI, RRF is relative response factor calculated by Ax*CISI(CS)/AISI*Cx(CS) from a 5 mg/L calibration standard (CS), and CF is a final concentration factor/reciprocal of the final dilution factor.
where Cx is the concentration of the target compound in the original sample, Ax is the measured peak area of the target compound in the sample extract, CISI is the spiked concentration of ISI, AISI is the measured peak area of ISI, RRF is relative response factor calculated by Ax*CISI(CS)/AISI*Cx(CS) from a 5 mg/L calibration standard (CS), and CF is a final concentration factor/reciprocal of the final dilution factor.
2.3.1.5. Inorganic Compounds by Inductively Coupled Plasma Optical Emission Spectrometry (ICP/OES):
The instrument consists of a 40 MHz plasma system with axial viewed plasma, a glass cyclonic spray chamber with a seaspray nebulizer, a high-resolution Echelle polychromator, and the charge coupled device detector for the simultaneous measurement of the full wavelength range from 167 to 785 nm. To obtain a final concentration of 1 mg/L Yttrium (Y, internal standard) and 2% HNO3 with a sample dilution factor of 1000/975, 19.75 mL of the sample solution was spiked with 0.25 mL of a solution containing 40 mg/L Y in 80% nitric acid. Finally, ICP measurement was conducted according to the conditions given below.
ICP/OES: ICP optical emission spectroscopy 720-ES (Varian, Inc.)
Target elements: Al, Ca, Fe, Mg, Si, S, Ti, Zn
2.3.1.6. Identification of Acrylic Acid Used as the Label Glue on PLAJEXTM:
About 1 mL of each test solution (PLAJEXTM) and blank (glass bottle) were transferred to 2 mL autosampler vials. Standard solutions of acrylic acid in water (ultra pure water) were prepared in the range of 1 to 1000 mg/L. These test solutions were measured by liquid chromatography/ultraviolet Spectroscopy (LC/UV).
Instrument: Agilent 1260 Infinity LC/DAD/FLD (Diode Array Detector/Fluorescence); Waters Symmetry Shield RP 18 column, 4.6 mm × 250 mm, 5 μm
LC Condition: Flow rate: 1 mL/min
Injection volume: 50 μL
Column temperature: 25 °C
Solvent: A:B = Acetonitrile:Sodium dihydrogen phosphate monohydride (pH = 2.73)
Methods: A% = 5% – (10 min) – 5% – (15 min) – 75% – (10 min) – 5%
Detector Wavelength: 200 nm
2.3.2. Analytical Method for Extractable Study:
2.3.2.1. Preparation of Analytical Samples for GC/MS and LC/MS:
To investigate the amounts of extractables from each component (glass barrel, COP barrel, BIIR plunger stopper, and CIIR plunger stopper), the analytical results of SVOC and NVOC of both systems detected by GC/MS were compared. The results of LC/MS were compared, as well as those of GC/MS.
2.3.2.2. SVOC by GC/MS:
The SVOC included in the extractables from WFI was analyzed by GC/MS under the conditions given below.
<GC/MS condition>
GC/MS: Agilent 7890B/5977A (Agilent Inc.)
Column: DB – 5 (30 m × 0.25 mm × 0.25 μm)
Injection temperature: 270 °C
Column temperature program: 50 °C (4 min) – (8 °C/min) – 300 °C (12 min)
Acquisition mode: Scan
Transfer line temperature: 300 °C
Ion source temperature: 230 °C
Quadrupole temperature: 150 °C
Full-scan range: 35–700 m/z
2.3.2.3. NVOC by LC/MS:
The NVOC included in the extractables from WFI was analyzed by LC/MS under the conditions given below.
<LC condition>
LC: Nexera (Shimazu Inc.)
Column: Waters Symmetry – C18 (4.6 mm × 250 mm, 5 μm)
Column temperature: 40 °C
Mobile phase and method: A) diluted water, B) methanol 0 min (B 40%) – 15 min (B 100%) – 90 min (B 100%)
<MS condition>
MS: Exactive (Thermo Fisher Scientific Inc.)
Ionization: APCI (+)/APCI (–)
Full-scan range: 100–1500 m/z
2.3.2.4. Inorganic Compounds by ICP/OES:
The extractables from WFI filled in the test samples were analyzed by ICP/OES under the conditions given below.
ICP/OES: ICP Atomic Emission Spectroscopy SPS5520 (Hitachi High-Tech Science Inc.)
Target elements: B, Ca, K, Mg, Na, S, Si, Zn
3. Results and Discussion
3.1 The Results and Discussion for Leachable Study (36 Months)
3.1.1. VOC and SVOC by HS-GC/MS and GC/MS Screening WFI:
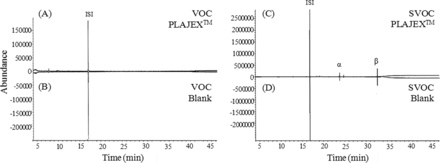
After 36 months' progress at 25 °C, contact (PLAJEXTM) solutions were taken and analyzed by HS-GC/MS and GC/MS. As a reference, the blank (glass bottle) solutions were taken and analyzed as well. The results of HS-GC/MS for the contact solutions and the blank solutions are shown in Figure 1. Figures 1(A) and (B) are charts of VOC for test and blank solutions and Figures 1(C) and (D) show charts of SVOC for test and blank solutions. As the results of VOC and SVOC analysis show, no differential compounds were detected above the reporting limit of 5 μg/L.
The chromatograms of the results of HS-GC/MS and GC/MS analysis for VOC and SVOC in the WFI filled into PLAJEXTM and blank (glass bottle) at 36 months. (A): VOC of PLAJEXTM, (B): VOC of blank, (C): SVOC of PLAJEXTM, (D): SVOC of blank (ISI: VOC; Toluene-d8, SVOC; 2-Fluorobiphenyl, α, β: error peaks).
3.1.2. NVOC by LC/MS Screening WFI:
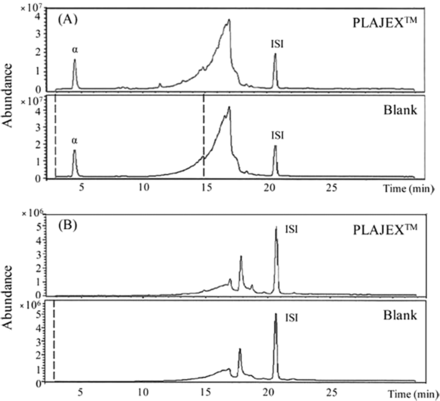
The test solution and blank (glass bottle) were also analyzed by LC/MS. Figure 2 shows the chromatograms of LC/MS analysis. Figure 2(A) represents the results of LC/MS analysis (APCI+) and Figure 2(B) shows the results of LC/MS analysis (APCI–) of the contact (PLAJEXTM) solutions and the blank (glass bottle) solutions, individually.
The chromatograms of the LC/MS analysis (A); APCI+ mode, (B); APCI– mode) in the WFI filled into PLAJEXTM (upper part of [A] and [B]) and blank (glass bottle) (lower part of [A] and [B]) (ISI; Tinuvin 327, α: error peak).
As a result, no differential compounds above the reporting limit of 3 μg/L were detected between contact and blank solutions at 36 months.
3.1.3. Leachable Compounds Identification:
Through the studies, some peaks were detected near the reporting limit levels in the organic leachable study and these peaks were identified and listed in Table III. We speculated that trimethylsilanol identified by GC/MS analysis were derived from degradation of the silicone oil component and N,N-Dibutylformamide and butylated hydroxytoluene were derived from antioxidants contained in the CIIR. The toxicity impact was estimated based on the toxic threshold calculated by a formula (body weight [50 kg], and the intravenous administration factor of safety). As a result, it was confirmed that the toxicity of the detected leachable compounds was much lower than toxic thresholds (data not shown).
The List of Detected Leachable Compounds in the WFI Filled into PLAJEXTM
In case of polymer-based PFS, it is a common concern that the glue on the label may permeate and migrate into syringes by aging. To confirm this, the amount of acrylic acid used as the label glue of PLAJEXTM was also analyzed separately with the test solution and blank solution (glass bottle) after 45 months' progress as the worst case.
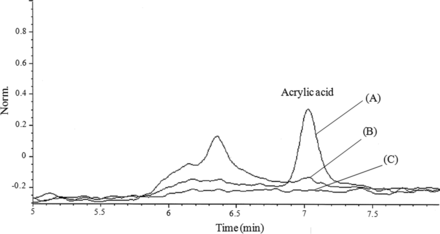
Figure 3 shows the results of LC/UV analysis of standard solution of acrylic acid used as label glue on PLAJEXTM (Figure 3[A]), contact (PLAJEXTM) solution (Figure 3[B]), and blank (glass bottle) solution (Figure 3[C]). The peak derived from acrylic acid was identified at 7.03 min, as shown in Figure 3. However, its peak was not detected in the contact solution. From the result of the leachable study, in conjunction with the additional analysis for acrylic acid, it was concluded that there was no label glue–related leachable migrated through the COP barrel into the drug solution. There are also no indications from the leachable study to indicate interference on the leachable profile from the ink applied on the label or from the secondary packaging.
The results of identification of acrylic acid migrated from the label glue on the PLAJEXTM barrel (A); standard solution of acrylic acid (B); PLAJEXTM (C); blank (glass bottle).
3.1.4. Inorganic Compounds by ICP/OES:
Inorganic elements that were eluted for WFI filled in PLAJEXTM and blank (glass bottle) were measured by ICP-OES (Table IV). Target elements (Ca, Mg, Si, S, Zn, Al, Fe, and Ti) were detected in both samples, but the amounts of detected elements were very low compared to the toxicity threshold. The amounts of detected Si from both PLAJEXTM and blank were increasing by aging, but the amounts of PLAJEXTM were higher than blank. Given this, we supposed that blank would be eluted Si compounds derived from the main ingredient of the glass bottle. On the other hand, Si compounds eluted from PLAJEXTM were derived from silicone oil on the barrel and stopper of PLAJEXTM. However, the amounts of detected Si eluted from PLAJEXTM does not have an influence of inherent toxicity.
ICP Results of Inorganic Elements Eluted for WFI Filled into PLAJEXTM and Blank (Glass Bottle)
3.2. Extractable Study
Through the various studies of the leachable study (HS-GC/MS, GC/MS, LC/MS, and ICP-OES), it was confirmed that there were no differences between the test solution and blank solution (glass bottle) even after 36 months' progress. In addition, no acrylic acid was detected in the test solution even after 45 months' progress as the worst case, as shown in Figure 3. These results are based on the PFS system as a whole. Therefore, it cannot refer to the individual components. For this reason, a further extractable study was conducted to identify the factors of the low extractable value of PLAJEXTM. In this study, the materials were selected from PLAJEXTM (COP barrel and CIIR plunger stopper). As a reference, conventional glass syringe components (glass barrel and BIIR plunger stopper) were selected.
3.2.1. Comparison Study between COP and Glass Barrel:
3.2.1.1. SVOC and NVOC by GC/MS and LC/MS screening WFI:
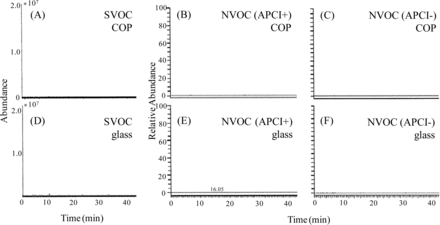
WFI extractions of the COP barrel and glass barrel were analyzed by GC/MS for SVOC and LC/MS for NVOC, individually. Figure 4 shows the results of GC/MS ([A] and [D]) and LC/MS ([B] - [C] and [E] - [F]) analysis. Of LC/MS analysis, positive and negative charged NVOC could be detected from APCI+ and APCI–, respectively. Based on this analysis, no compounds were detected above the reporting limit of 5 μg/L.
The chromatograms of the results of GC/MS and LC/MS analysis for SVOC and NVOC in the WFI filled into COP and glass barrel. (A): SVOC of COP, (B): NVOC (APCI+) of COP, (C): NVOC (APCI–) of COP, (D): SVOC of glass, (E): NVOC (APCI+) of glass, (F): NVOC (APCI–) of glass.
3.2.1.2. Inorganic Compounds by ICP/AES Screening WFI:
Table V shows the result of WFI extractions of the COP barrel and glass barrel analyzed by ICP-AES. As shown in Table V, Na and Si were detected from WFI extractions from the glass barrel. It is thought that Na and Si were generated from glass main ingredients (Na2O-B2O3-SiO2). On the other hand, no elements were detected from WFI extractions in the COP barrel.
Extracted Amount of COP and Glass Barrel Measured by ICP-AES
3.2.2. Comparison Study between CIIR and BIIR Plunger Stoppers:
3.2.2.1. SVOC and NVOC by GC/MS and LC/MS screening WFI:
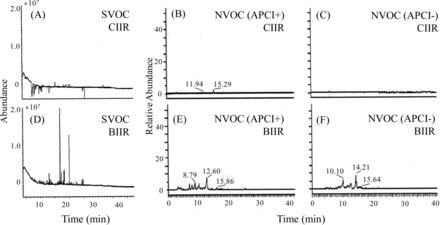
WFI extractions of CIIR and BIIR plunger stoppers were analyzed by GC/MS for SVOC and LC/MS for NVOC, individually. Figure 5 shows the data after these GC/MS or LC/MS data were subtracted from the blank data. The results of GC/MS are shown in Figures 5(A) and (D), the results of LC/MS (APCI+) are shown in Figures 5(B) and (E), and the results of LC/MS (APCI–) are shown in Figures 5(C) and (F). Compared to the CIIR plunger stopper, it was confirmed that many peaks were observed in the BIIR plunger stopper in the chromatograms of both GC/MS and LC/MS. From these results, it is clear that the extractables from the CIIR plunger stopper were lower than those from the BIIR plunger stopper.
The chromatograms of the results of GC/MS and LC/MS analysis for SVOC and NVOC in the WFI filled into CIIR and BIIR plunger stopper. (A): SVOC of CIIR, (B): NVOC (APCI+) of CIIR, (C): NVOC (APCI–) of CIIR, (D): SVOC of BIIR, (E): NVOC (APCI+) of BIIR, (F): NVOC (APCI–) of BIIR.
3.2.2.2. Inorganic Compounds by ICP/AES screening WFI:
Table VI shows the results of WFI extractions of CIIR and BIIR plunger stoppers analyzed by ICP-AES. As shown in Table VI, Na and Si were detected from WFI in the CIIR plunger stopper. On the other hand, Mg and S, in addition to Na and Si, were detected from WFI in the BIIR plunger stopper. These results also indicated the elemental extractables in BIIR are higher than in CIIR, as do the results of organic extractables shown in Figure 5.
Extracted Amount of CIIR and BIIR Plunger Stopper Measured by ICP-AES
In general, sulfur compounds and MgO were used during the manufacturing process of BIIR to create cross-linkage. On the other hand, in the case of CIIR, these compounds were not used. Therefore, the differences in extractables between this CIIR and BIIR might be due to the difference in the formulation composed of rubber.
Through the extractable study, it was confirmed that the extractable amounts in the COP and the glass barrel were similar in terms of SVOC and NVOC comparison study. In the ICP-AES comparison study, no elements were detected from the COP barrel, but Na and Si were detected from the glass barrel. In addition, the extractable amount from the CIIR plunger stopper was lower than that of the BIIR plunger stopper in terms of the SVOC, NVOC, and ICP-AES comparison studies. From these findings, it was demonstrated that lower extractables of PLAJEXTM were achieved by selecting materials and/or components with lower extractables.
4. Conclusion
The result of the leachable study of WFI-filled PLAJEXTM after 36 months' progress confirmed that no remarkable leachables were detected, and the result was the same as for the glass bottle used as the reference control sample. In addition, a label glue migration study was also conducted with WFI-filled PLAJEXTM after 45 months' progress. As a result, no acrylic acid from the glue was detected. To identify the reason for the low extractable values, a further extractable study was conducted with several key components. As a result, it was concluded the reason for the low extractable value of PLAJEXTM came from both COP and the applied CIIR. In addition, the applied CIIR was clearly superior to BIIR in terms of extractables.
In conclusion, the proposed PLAJEXTM composed of a COP barrel and CIIR plunger stopper has great potential and represents a novel alternative that can achieve very low levels of extractables and can bring additional value to the highly sensitive biotech drug market.
Acknowledgements
The authors gratefully acknowledge Toxikon Europe NV and Sumika Chemical Analysis Service, Ltd., for their professional contribution, support, and expertise. They have responded to our requests patiently and provided their analytical services to our fullest satisfaction.
- © PDA, Inc. 2015