Abstract
The manufacturing processes of biological medicinal products are expected to be capable of removing and/or inactivating viruses, to primarily provide for adequate safety margins to patients and to thus satisfy the corresponding regulatory expectations. To achieve this goal, process segments specifically dedicated to the task of virus removal or inactivation are designed into manufacturing flows. The state of the art now includes virus removal by nanofiltration, and the more traditional low pH or (solvent-) detergent treatments are used widely, often to provide for two complementary and mechanically different means of virus clearance. Reflective of these process preferences, the virus-filtration/-inactivation session included seven case studies on virus filtration, and one each for a detergent and a low pH treatment.
LAY ABSTRACT: To enhance virus clearance capacity, manufacturing processes of therapeutic proteins include process steps dedicated to virus clearance, especially virus-retentive filtration and virus inactivation. This article summarizes the current understanding of process preferences for the said process steps.
- Downstream processing
- Virus-retentive filtration
- Virus inactivation
- Low pH treatment
- Detergent treatment
Introduction
The manufacturing processes of biological medicinal products are expected to be capable of removing and/or inactivating viruses, to primarily provide for adequate safety margins to patients and to thus satisfy the corresponding regulatory expectations. To achieve this goal, process segments specifically dedicated to the task of virus removal or inactivation are designed into manufacturing flows. The state of the art now includes virus removal by nanofiltration, and the more traditional low pH or (solvent-) detergent treatments are used widely, often to provide for two complementary and mechanically different means of virus clearance. Reflective of these process preferences, the virus-filtration/-inactivation session included seven case studies on virus filtration, and one each for a detergent and a low pH treatment.
Optimization of Viral Filtration Process Conditions to Enable a Representative Scale-Down Model
Michael Clark, AbbVie
Laboratory-scale virus-filtration models can have limited throughput compared to large-scale manufacturing processes, creating a challenge for validation of the process and may result in underutilization of the true filter capacity and thus unnecessary process costs. In the presented example, freezing and thawing of the load material at a small scale, which is not representative of the manufacturing process, resulted in limited throughput. To address the limitation, the process buffer composition for viral filtration load was optimized.
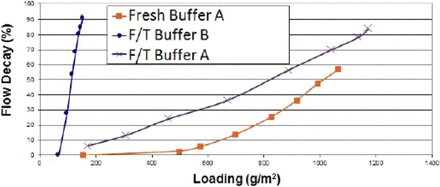
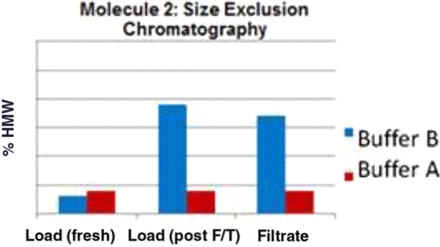
Buffer A and Buffer B represent the new and previous platform buffer compositions, respectively. “Fresh” indicates that the virus-filtration load was never frozen, while “F/T” indicates that the load was frozen and thawed prior to filtration. The data presented show that for the F/T load materials, Buffer A allows much higher filtration throughput than Buffer B (Figure 1 shows one of the five molecules studied). A similar trend was observed for all five molecules. Determination of high-molecular-weight (HMW) species versus buffer composition, as well as whether the process stream had undergone freezing and thawing, revealed that for Buffer A, there is no appreciable change to HMW species content following F/T, whereas a notable increase in the number of HMW species was observed when Buffer B was used (Figure 2). Additionally, the use of an adsorptive prefilter offered a moderate throughput improvement with Buffer A, although no significant improvement was noted for Buffer B as illustrated in Figure 3.
Impact of freeze/thaw and buffer composition on throughput.
Impact of freeze/thaw and buffer composition on throughput.
Impact of buffer composition on throughput.
Virus Filtration—challenges for Scale-Down
Helmut Winter, BI
Virus filtration is a robust virus removal step, although low pressure and flow-pause events during manufacturing have been recognized to potentially result in lower reduction factors for parvoviruses (1⇓–3).
In the presented generic study, virus-spiked filtration runs at standard worst-case conditions, including maximum load volume (+5%–10%), maximum pressure (+0.1 bar), maximum wash volume (+10%–20%), and a pressure release between end of load and wash for ≥10 min were compared with runs at low pressure and with up to 15 filtration stops to simulate unplanned process conditions.
As summarized in Table I, all virus filtrations under standard worst-case conditions showed complete virus reduction, in phosphate (P) and acetate (A) buffers. The runs mimicking unplanned process conditions resulted in >5 log10 virus removal, although for certain conditions, virus removal was not complete.
Impact of Buffer System and Flow Conditions on Virus Reduction
Effects of Pressure Source on Virus Breakthrough During Viral Filtration
Sean O'Donnell, Eli Lilly
As mentioned earlier, operating conditions during virus filtration could potentially affect virus retention. The viral filtration unit operation at Lilly is performed under constant pressure across the filter, with either compressed air or a pump-driven system as the pressure source. To determine whether different pressure sources could potentially influence the effectiveness of virus retention, a systematic comparison was carried out using three commercially available viral filters, including 30-min pressure pauses during filtration and before the filter wash.
For the three filters investigated, all runs resulted in complete removal of porcine parvovirus, for all the individually titrated filtrate/wash fractions. Results from the study thus support a flexible choice of pressure source, which may be important in the context of facility fitting and unit operation control in Good Manufacturing Practices (GMP) production.
Multiple, Deliberate Interruptions of a Virus Removal Filtration Process—Impact on Virus Retention and Throughput
Konstantin Zoeller, Novartis
Published data suggest that hydrodynamic forces influence virus retention for small viruses by limiting particle movement in the filter membrane at high flow rates (4, 5). The interruption of flow during the nanofiltration process is therefore regarded to be one of the reasons for increased passage of small virus across the nanofilters, although the virus passages reported did not severely reduce viral clearance capacity of the overall process.
In the presented work, a Viresolve® Pro (EMD Millipore Corporation, Billerica, MA, USA) nanofilter was loaded with a minute virus of mice (MVM)-spiked protein solution or buffer, to investigate if there is a different behavior in the presence or absence of protein. The experiment was performed at a constant flow rate, and the delta pressure was monitored. During five interruptions of at least 3 h (total elapsed time), the flow rate across the filter was reduced to zero, and fractions for virus titration were collected after every interruption and analyzed by large volume plating. After the first and last interruption, smaller fractions were taken to increase the chance to find a virus particle passing the filter directly after interruption.
Despite the interruptions, filtration throughput was maintained (data not shown). For all the fractions collected, virus removal to below the detection limit was shown, for the buffer and the protein solution experiments (Table II), which shows that the virus removal capacity of Viresolve Pro is not sensitive to limits of hydrodynamic force under the conditions tested. The data obtained suggest that the filter design of Viresolve Pro offers high virus-retentive capacity independent of process interruptions. This observation confirms the results of Dishari et al.'s (6) study, which looked directly at fluorescence-labeled phage distribution in a Viresolve Pro membrane with confocal microscopy. Diffusion effects were observed that were directed more toward the inlet of the filter rather than the outlet. Nonetheless, the process conditions including planned interruptions in a viral clearances study should be suitably tested as outlined in ICH Q5A. This study, however, may be limited to parvoviruses only as the worst case in terms of virus size for 20-nm membranes.
Impact of Flow Interruptions on Virus Reduction
Validating an Alternative Virus Reduction Filter for a Commercial Product
Dennis Twomey, Janssen
As part of a cost-efficiency initiative and standardization with a platform virus-filtration process step, an alternative virus-filtration filter for an approved commercial process was validated. The model viruses used to support the validation claims for the alternative filter were different from the panel of viruses used for the original commercial process. In addition to the use of a cell line–specific model virus, xenotropic murine leukemia virus (X-MuLV), viral clearance studies using murine minute virus (MVM) were performed, replacing poliovirus as a worst-case small model virus because of the smaller size of MVM and international initiatives.
Based on platform knowledge and experience, a design-of-experiment (DOE) approach to virus clearance studies was used to support the validated virus clearance claims for the alternative filter. A series of eight reduced-scale virus clearance runs were executed at a filter load of approximately 1200 g/m2, evaluating the impact of low and high protein concentration and low and high filtration pressure on the virus reduction claims. Although not part of the routine processing, the studies also incorporated a process pause in the study design to evaluate short process pauses that may occur unintendedly in the manufacturing process.
The virus clearance data generated support minimum log10 reduction values (LRV) of >4.11 for X-MuLV and 4.24 for MVM (Table III), with MVM worst-case conditions at low pressure (0.53 bar), that is, consistent with the reported breakthrough under low pressure conditions.
Impact of Protein Concentration and Differential Filtration Pressure on Virus Reduction
These data complemented the overall validated virus clearance claims of the commercial process.
The implementation of the alternative virus-filtration filter for an approved commercial process has provided a more cost-effective process with robust process performance.
Investigational Study on the Robustness of the 20-nm Nanofiltration Step Performed According to the Doe Methodology
Céline Ducloux, LFB
Nanofiltration of plasma-derived medicinal products is particularly relevant owing to its effectiveness in removing small, nonenveloped viruses such as hepatitis A virus (HAV) and parvovirus B19V. Its implementation for HMW protein solutions such as Immune Globulin G (IgG) and some clotting factors can be challenging though. A DoE approach was used to evaluate the effectiveness of porcine parvovirus (PPV) removal by double or 20-20 nm of nanofiltration for a plasma product, to define a design space within which the critical quality attribute (CQA) viral safety is achieved. Based on process understanding, product concentration, pressure, temperature, and volumetric load were defined as critical process parameters (CPPs) with a potential impact on virus removal by nanofiltration.
The DoE was successfully used to identify a design space within which the CQA viral safety was confirmed, with either complete (>4 LRV) or very effective (=5 LRV) PPV removal (Table IV). Any future manufacturing process changes will require much less effort, so long as the process remains within the already validated and approved design space.
Design of Experiments Approach to Evaluate Virus Filtration
What to Do When the Virus Filtration Cannot Be Validated as Usual?
Thilo Grob, Rentschler
According to current regulatory guidelines, the mechanism of the virus removal and/or inactivation has to be unequivocally determined to support claims of orthogonal steps for the overall virus reduction capacity of the process. Specific process requirements or conditions during the virus filtration can make it challenging to quantify the virus removal capacity by size-exclusion mechanisms; for example, when the load material contains a virus-inactivating agent (e.g. caprylate) or is inclined to generate aggregates or precipitates, an adsorptive prefilter is thus needed to support the processing of an adequate volumetric load.
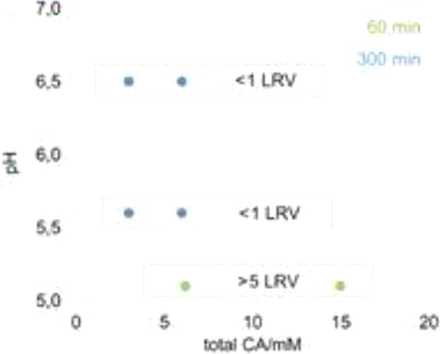
It is known that only the deprotonated caprylate (CA) form bears the potential to inactivate viruses. Figure 4 illustrates the inactivation potential of CA depending on concentration and pH value. It clearly shows that CA does not inactivate viruses at pH 5.5 and higher. A pH adjustment step of the virus filter load in the routine manufacturing process may solve the virus validation issue. However, this approach is contradictory to the general approach to keep the manufacturing process as simple as possible.
Virus inactivation of MuLV by CA depending on concentration and pH.
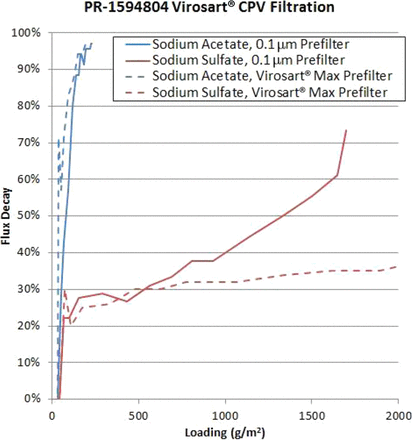
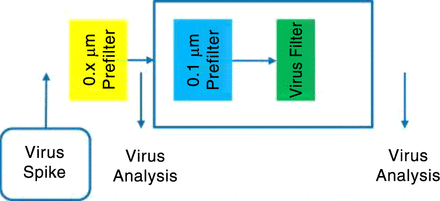
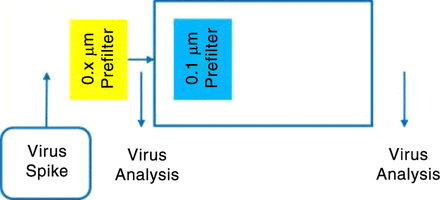
In-line prefiltration before the virus filter is mandatory for some processes (Figure 5). In most cases, a functionalized prefilter is used for this application. The kinetics of the formation of aggregates or precipitates occur very fast such that an offline filtration is not feasible. A nonacceptable, low maximum load on the virus filter would be the result. To validate such a process, virus removal by the 0.1-μm prefilter has to be investigated (Figure 6). For such processes, a prefilter with a hydrophobic characteristic is successfully used at Rentschler.
Virus validation when prefiltration is mandatory.
Additional virus removal experiment for the prefilter.
In one example presented (Table V), the enveloped viruses X-MuLV) and pseudorabies virus (PRV) were already substantially removed by the required prefilter with hydrophobic characteristics, whereas the nonenveloped MVM was not significantly retained.
Impact of Prefiltration on Virus Reduction
Because the mechanism of virus retention by the hydrophobic prefilter cannot be unambiguously determined, it was deduced that only the combination of prefilter and virus filter could be claimed for overall virus clearance. As a consequence, it may not be possible to claim other steps on the basis of hydrophobic interaction for overall virus reduction, such as mixed-mode resins, which may have good virus removal capacity.
A suggested resolution for such experimental limitations may be claiming of MVM data for virus removal in general on the basis of their smallest size reflecting a worst-case challenge for virus-retentive filters anyway (7).
Establishing a Generic Claim for Lauryldimethylamine N-oxide Detergent Inactivation
Brad Stanley, Biogen
As part of a comprehensive viral safety program, Biogen sought a framework within which robust detergent inactivation can be ensured in a cell culture fluid. Previous work has shown lauryldimethylamine N-oxide (LDAO) inactivation as robust across a wide range of antibodies, protein concentration, pH, conductivity and host cell impurities in cell-free harvest, with a negative effect on inactivation observed at only lower detergent concentrations (8).
To address the potential concern of process-related impurities that could possibly adsorb detergent, such as lipids or other lipophilic components, the current study showed complete inactivation of X-MuLV as model lipid-enveloped virus in the presence of high concentrations of host cell lipids (i.e. palmitoleic and oleic acid, the two predominant lipids in harvest, and/or antifoam), Table VI.
Impact of Lipids and Antifoams on Detergent Inactivation of X-MuLV
Based on virus validation data from over 10 molecules and on robustness data extending well beyond expected ranges for pH and conductivity, as well as process-related impurities such as proteins, lipids, and antifoam, a modular claim for LDAO inactivation of cell culture harvest is proposed for LDAO concentrations of >0.14%, incubation time for >60 min at >2°C, and pH 6–8, which is very similar to the ASTM (American Society for Testing Materials) standard practice for Triton X-100 inactivation (E3042-16).
Virus Inactivation at Low pH: Impact of Buffer Composition, pH Value, and Protein Concentration on the Inactivation of Different Retroviruses
Franz Nothelfer, BI
Incubation at low pH is a widely used virus-inactivation step for biotechnology processes, and it is typically investigated for effectiveness using murine leukemia virus (MuLV) as a model virus. The virus-inactivation results reported have not been fully consistent, despite apparently identical pH values and incubation times. As to the underlying reasons, speculations have included differences between contract laboratories, strains of MuLV, buffer salts, and protein concentrations.
The current case study experimentally investigated the potential impact of different buffer compositions, protein concentrations, and pH values on the inactivation of different retrovirus models—in other words, amphotropic MuLV (aMuLV), Moloney (Abelson) leukemia virus (Mo/A-MuLV), gibbon ape leukemia virus (GaLV), and X-MuLV.
As can be seen in Table VII, X-MuLV and Mo/A-LV were found the most sensitive retrovirus models at pH 3.6, with complete virus inactivation under all conditions tested. The inactivation of aMuLV and GaLV at pH 3.6 was, however, dependent on the buffer salt system, with acetate more effective than citrate and much more effective than glycine/phosphate buffer, and higher protein concentrations also resulted in lower virus inactivation.
Low pH Inactivation of Retroviruses in Different Buffer Systems
Conclusion
During the session, the key contributing parameters to viral filtration performance were discussed. The data presented indicate that freeze/thaw (F/T) of samples prior to viral filtration (VF) can lead to self-association and hence affect performance. Therefore, care should be taken to evaluate the potential impact of F/T on process performance. Two additional parameters with the potential to affect VF performance are buffer composition and the use of an in-line prefilter, which, in some cases, can reduce turbidity and improve VF loading. However careful consideration of the use of a prefilter is necessary, as the prefilter may also remove virus by multiple mechanisms (size and adsorption) and hence an integrated approach (prefilter/virus filter) should be evaluated. It should be noted that the impact of changes of buffer composition on self-association and the tools to assess these changes have been discussed at the 2013 Viral Clearance Symposium (9) and subsequently, in the literature (10).
There was significant discussion in the session regarding the potential differences between scale-down and production viral filtration processes including an assessment of worst-case conditions. The recommendation was to link the small-scale experimental design to include key parameters such as pressure/pauses and duration in the scale-down experiments. Results presented for specific case studies suggest that the virus passage does not occur under multiple pressure/pause scenarios or when operating the filter under typical worst-case conditions (e.g., low pressure, high loadings) and with MVM as the model virus owing to the small size. Further investigation is recommended to understand the mechanisms of potential virus passage under low flow, low pressure, and fouling conditions (high-load conditions). Key parameters that may affect breakthrough include viscosity of the solution, protein concentration, virus spike level, membrane loading, pH of the solution, and interactions (e.g., ionic or hydrophobic) between the virus and the membrane. The potential impact of the duration of the pause (e.g., 10–60 min) and the rate of the pressure pause change were parameters that may warrant further assessment. The effect of the majority of these parameters (11) including for specific filters (12) has been explored in the literature. The combination of the date presented in the session and recent publications on the origin and incidence of virus passage suggests that virus passage may be a limited-frequency occurrence and the potential can be systematically evaluated.
Case studies presented in the session showed the feasibility of using the combination of a DoE and reduced the experimental data set as part of a Quality by Design (QbD) approach to viral filtration. This approach has potential for process development or for the assessment of the impact of changing a viral filter in a commercialized process.
The session concluded with an overview of use of low pH for virus inactivation. The outcome of the discussions was that there is currently general acceptance of the ASTM standard (E2888). However, there have been differences reported in the inactivation extent (LRV) observed in various buffer systems—complete inactivation observed in acetate, whereas more limited inactivation in glycine and phosphate systems. Although X-MuLV is typically used as the model virus for inactivation studies, data presented during the session suggest that different retroviruses may have differential inactivation levels. This suggests that X-MuLV may be a best-case model. Follow-up experiments to understand the effect of the origin of the differences in the observed inactivation rates in various buffers and as a function of the type of virus would be beneficial.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2018