Abstract
In our previously published work, we reported rapid polysorbate 80 (PS80) oxidation in a histidine buffer after brief exposure to stainless steel and the ability of citrate and EDTA to prevent this oxidation. The focus of our current study was to mechanistically understand PS80 oxidation by studying the impacts of temperature, light, and stainless steel and the role of citrate and EDTA. Additionally, PS80 oxidation was studied in three different buffer systems: histidine, citrate, and phosphate. When the PS80-containing buffers in glass containers were exposed to the elevated temperature of 50°C, no PS80 oxidation was observed in either the histidine or the citrate buffer systems after 30 days; however, PS80 oxidation was observed in the phosphate buffer system within 14 days. These results demonstrated that temperature does not initiate PS80 oxidation in the histidine or the citrate buffer systems, but it may be a factor in the phosphate buffer system. When the three buffer systems containing PS80 were exposed to 20%, 50%, or 100% ICH Q1B light conditions and subsequently incubated in the dark at 50°C, the PS80 in the phosphate buffer system underwent oxidation within 7 days, whereas the PS80 in the histidine and the citrate buffer systems showed oxidation products only after 14 and 35 days, respectively. PS80 in the phosphate buffer system seemed to be the most vulnerable to light as PS80 in both the histidine and the citrate buffer systems underwent oxidation to a lesser extent, with faster oxidation occurring in the histidine buffer system than in the citrate buffer system. Finally, the ability of citrate and EDTA to act as not only chelators but also radical quenchers/scavengers was demonstrated when a metal ion, Fe2+, was spiked into the histidine buffer containing PS80. While radicals could not be unambiguously identified by NMR or EPR, the observation of PS80 oxidation products indicated their presence.
LAY ABSTRACT: In our previously published work, we reported rapid polysorbate 80 (PS80) oxidation in a histidine buffer after brief exposure to stainless steel and the ability of citrate and EDTA to prevent this oxidation. The focus of our current study was to mechanistically understand PS80 oxidation by studying the impacts of temperature, light, and stainless steel and the role of citrate and EDTA. Additionally, PS80 oxidation was studied in three different buffer systems: histidine, citrate, and phosphate. The temperature study demonstrated that PS80 oxidation in the histidine or the citrate buffer systems is not initiated by temperature, but may be a factor in the phosphate buffer system. PS80 in the phosphate buffer system seemed to be the most vulnerable to light, as PS80 in both the histidine and the citrate buffer systems underwent oxidation at a lower level, with the histidine buffer system showing more rapid oxidation than the citrate buffer system. Finally, the ability of citrate and EDTA to act as not only chelators but also radical quenchers/scavengers was demonstrated when a metal ion, Fe2+, was spiked into the histidine buffer containing PS80. While neither NMR nor EPR could definitively identify the presence of free radicals, the observation of PS80 oxidation products indicates that they were present.
Introduction
Polysorbate 80 (PS80) is a nonionic surfactant commonly used in the pharmaceutical industry to protect and stabilize drug products from different stresses that would otherwise lead to aggregation of active protein (1⇓⇓⇓–5). It is well known that PS80 undergoes degradation by both oxidation and hydrolysis and that its oxidative degradation may be accelerated by different initiators such as temperature, light, and stainless steel (6⇓⇓⇓–10). In our previously published work (11), we reported rapid PS80 oxidation in the histidine buffer after brief exposure to stainless steel and the ability of EDTA to prevent this oxidation likely because of its metal chelating capabilities. We also reported the ability of protein (at higher concentrations) to quench the formed free radicals. Further, we showed that the PS80 degradation was mostly driven by metal-induced oxidation rather than by headspace oxygen. In addition, the previous study also demonstrated that the oxidation was dependent on the type of buffer system, with rapid PS80 oxidation occurring in the histidine buffer system and little-to-no oxidation occurring in a citrate buffer system at the same temperature even after stainless steel exposure (11). It is known that when citrate is added as a buffer species to maintain the pH of a formulation it can also act as a weak chelator, which explains the lack of oxidation observed in previous studies (12⇓–14). Ethylenediaminetetraacetic acid (EDTA) is a strong chelator and is widely used in formulations to chelate trace metals. Trace levels of metals are often contributed by formulation excipients, manufacturing contact surfaces, and package components (15). While metal ions play an important role in the biological functions of many proteins, excessive amounts of metal ions, such as iron, can lead to protein aggregation (15) and enhance PS80 oxidation (15). A previous study showed that a histidine buffer containing 0.06% PS80, 100 ppm EDTA, and 5 ppm Fe2+ at 25°C was stable for up to three months with no oxidation of PS80, but a sample lacking EDTA was stable for only three weeks at the same temperature (11). The focus of the current study was threefold:
Understand the mechanism of PS80 oxidation when in contact with stainless steel (316) surfaces.
Identify and characterize the different free radicals formed as a function of temperature, light, and stainless-steel contact and assess PS80 oxidation as a function of the buffer species.
Compare the chelating/free radical quenching capabilities of citrate and EDTA in the presence of spiked iron and stainless-steel contact.
Materials
Materials were purchased as follows: L-histidine (USP/Ph. Eur./JP) and L-histidine hydrochloride monohydrate (Ph. Eur./JP) from Kyowa Hakko (Hofu City, Japan); sodium phosphate monobasic monohydrate (USP) and polysorbate 80 (NF, JP & Ph.Eur.) from Avantor Performance Materials (Phillipsburg, NJ); sodium phosphate dibasic anhydrous (USP) from Ellis & Everard (Widnes, England); sodium citrate dehydrate (USP, JP & Ph.Eur.) from A & C American Chemical (Quebec, Canada); citric acid anhydrous (USP, JP & Ph.Eur.) from Jungbunzlauer Canada Inc. (Ontario, Canada); 5 and 50 mL borosilicate glass vials and bromobutyl serum stoppers from the West Company (Omaha, NE); Na2EDTA from J.T. Baker (Paris, KY); ferrous sulfate heptahydrate from Sigma Aldrich (St. Louis, MO); deactivated 12 × 32 mm glass snap cap vials from Waters (Milford, MA); Sanyo incubator from Sanyo North American Corporation (Tokyo, Japan); SUNTEST XLS+ light chamber from Atlas Material Testing Solutions (Mt. Prospect, IL); 5 mm NMR tubes from Wilmad-LabGlass (Vineland, NJ); deuterium oxide “100%” from Cambridge Isotope Laboratories, INC (Tewksbury, MA); fluorinated spin trap, FDMPO, from Enzo Life Sciences (Farmingdale, NY); and methyl sulfoxide-d6 for NMR from Acros Organics (Geel, Belgium).
Methods
Liquid Chromatography–Mass Spectrometry (LC-MS) to Detect PS80 Oxidation
Oxidation of PS80 was monitored using a Waters Acquity UPLC coupled to a Waters Synapt G2-Si mass spectrometer. One microliter of undiluted sample was injected onto a PLRP-S reversed-phase column (1 × 50 mm, 300 Å, 5 µm), previously equilibrated for 1 min in 37% mobile phase B (0.04% trifluoroacetic acid [TFA] in acetonitrile) and 63% mobile phase A (0.05% TFA in water), at 0.6 mL/min. The separation gradient increased linearly for 5 min from 37% mobile phase B to 100% mobile phase B at a flow rate of 0.3 mL/min. It was then held at 100% mobile phase B for 1 min before re-equilibration at 37% mobile phase B. Analytes eluting between 1 and 4.6 min were ionized via an electrospray ionization (ESI) source in positive mode. The scan range was 180–2000 Da with a capillary voltage of 3.2 kV and a capillary temperature of 150°C. The cone voltage was 100 V with a desolvation temperature of 500°C. Intact PS80 was quantified using a calibration curve, with PS80 standard concentrations ranging from 0% to 0.03% PS80. Standards were prepared using a 1% PS80 stock solution diluted with water. The concentration of intact PS80 at each time point was estimated based on the peak area of the extracted chromatogram of the dioxalanylium ion of PS80 oleate. In addition, oxidation was determined based on the presence or absence of the peaks in the extracted chromatograms of the dioxalanylium ions for the given PS80 oxidation products.
Nuclear Magnetic Resonance (NMR) for Radical Trapping Experiments
All NMR experiments were conducted on an Agilent DD2 600 MHz spectrometer equipped with an Agilent 1H-19F/15N-31P PFG OneProbe. 19F-NMR spectra were acquired at room temperature with the number of scans set to 512. A 100 mM sample of 4-hydroxy-5,5-dimethyl-2-trifluoromethylpyrroline-1-oxide (FDMPO) stock was prepared in methyl sulfoxide-d6. Aliquots of samples were prepared in 10% D2O (v/v) and spiked with 5 mM FDMPO to produce a total final volume of 600 µL. Chemical shifts were referenced by setting the unreacted FDMPO to −66.0 ppm.
Electron Paramagnetic Resonance (EPR) for Radical Trapping Experiments
A Bruker EMXplus EPR spectrometer was used at the University of Kansas NMR Core Lab to trap free radicals in stability samples. The spectrometer was operated at an X-band microwave frequency of 9.8 GHz with a high-sensitivity probehead cavity resonator. Samples (335 µL) were spiked with 15 µL of 150 mM spin trap 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO), of which 300 µL was then placed onto a clean, dry, Suprasil aqueous flat cell (Wilmad-LabGlass p/m WG-812-Q). The sample was then inserted into the EPR, tuned, and then analyzed at room temperature.
Experimental
The impacts of temperature, light, and stainless-steel contact on PS80 oxidation were studied. In addition, the formed free radicals were trapped using trapping agents and analyzed by NMR/EPR.
Impact of Temperature
The impact of temperature on 10 mM histidine, citrate, and phosphate buffers containing 0.02% (w/v) PS80 at pH 5.5 was assessed by storing them in glass containers and exposing them to 50°C for up to 30 days. A 3 mL aliquot of each buffer was taken after 0, 1, 3, 7, 14, and 30 days and tested for PS80 degradation by LC-MS.
Impact of Light
The impact of light on 10 mM histidine, citrate, and phosphate buffers containing 0.02% (w/v) PS80 at pH 5.5 in glass vials was assessed by exposing these to 20%, 50%, and 100% ICH Q1B light using a SUNTEST XLS+ light chamber and subsequently storing them in the dark at 50°C after light exposure. The time 0 in this experiment was defined as the time after exposure to the various light conditions but before storage at 50°C. A 3 mL aliquot of each buffer was taken at time 0 and after 1, 3, 7, 14, and 35 days of storage at 50°C and was tested for degradation of PS80 by LC-MS. In addition, a dark control of each buffer solution was generated by wrapping a glass vial containing the buffer in foil and storing it in the light chamber. The dark control samples allowed differentiation of the degradation caused by light from the degradation caused by temperature alone. Aliquots of the dark control were taken at the same time points and analyzed in the same way as the light-exposed samples and PS80 degradation was determined by LC-MS.
Impact of Contact with Stainless Steel (316)
Whereas PS80 oxidation in a histidine buffer upon brief contact with stainless steel has been previously reported (11), the current study also included citrate and phosphate buffers. The impact of contact with a 316 stainless-steel surface was studied using 10 mM histidine, citrate, and phosphate buffers containing 0.02% (w/v) PS80 at pH 5.5. The buffers were exposed to stainless steel at 50°C for up to 30 days. Aliquots of each buffer (3 mL) were taken after 0, 1, 3, 7, 14, and 30 days and tested for PS80 degradation by LC-MS. The vials from the impact of temperature study were used as controls for this study.
Mechanistic Understanding of the Function of Citrate and EDTA in Preventing PS80 Oxidation:
The ability of citrate and EDTA to protect PS80 from oxidation in a histidine buffer, which was reported in our earlier work (11), was further studied here to elucidate the mechanism by which PS80 oxidation is prevented. Our hypothesis leading into this study was that citrate and EDTA either chelate the metal ions responsible for generating these free radicals or are responsible for quenching the formed free radicals, thereby protecting PS80 from oxidation in a histidine buffer system. A 1 M citrate buffer stock at pH 5.5 was prepared, and an EDTA stock solution at 0.5 M was used for this study. Eleven 25 mL aliquots of the 10 mM histidine buffer containing 0.02% PS80 were prepared in stainless-steel containers and spiked with the citrate stock such that the citrate concentrations in the final solutions were 0, 0.0625, 0.5, 1, 25, and 100 mM. Likewise, buffer solutions were spiked with EDTA stock solutions such that the final EDTA concentrations were 0, 1, 10, 100, and 1000 µM EDTA. The spiked samples were incubated in stainless steel at 50°C for 24 h and subsequently transferred into 50 mL glass vials and further incubated at 50°C for up to 25 days. The point at which the samples were transferred from the stainless steel to the glass was defined as time 0. Aliquots (3 mL) of each citrate- and EDTA-spiked sample were taken at 0, 2, 7, and 25 days and tested for PS80 degradation by LC-MS.
In order to test the hypothesis about the ability of citrate and EDTA to quench the formed free radicals, a similar study was conducted where the citrate buffer and the EDTA were spiked (between 0 and 1000 µM) into the histidine buffers after the buffers were exposed to stainless steel at 50°C for 24 h. Essentially, eleven 25 mL aliquots of the 10 mM histidine buffer with 0.02% PS80 were prepared in stainless steel and incubated at 50°C for 24 h. After incubation, the samples were transferred to glass and spiked with the citrate buffer stock to ensure 0, 0.0625, 0.125, 0.25, 0.5, or 1 mM citrate concentration or with the EDTA stock to ensure 0, 1, 10, 100, or 1000 µM EDTA concentration in the solutions. The period of post-spiking with either the citrate or the EDTA stock was defined as time 0. Three milliliter (3 mL) aliquots were taken from each sample solution at 0, 1, 7, 14, and 24 days (for citrate) and at 0, 1, 7, and 25 days (for EDTA) and tested for PS80 degradation by LC-MS.
Iron Spiking in Addition to Stainless-Steel Contact Study:
The ability of citrate and EDTA to chelate spiked iron was studied in the 10 mM histidine buffer with 0.02% PS80 (w/v). A 500 ppm Fe2+ stock solution, a 0.5 M EDTA stock solution, and a 1 M citrate buffer stock solution were used. Twelve 25 mL aliquots of the histidine buffer containing PS80 were prepared in 50 mL stainless-steel containers. Four of these aliquots were spiked with citrate such that the final concentration was 62.5 µM citrate; likewise, four aliquots were spiked with EDTA such that the final EDTA concentration was 62.5 µM, and the remaining four aliquots contained neither citrate nor EDTA. All 12 aliquots were spiked with Fe2+ such that the final Fe2+ concentrations were 0, 0.1, 1, and 10 ppm. All samples were incubated at 50°C for 24 h in stainless steel and then transferred to glass for further incubation at 50°C. The time of transfer from the stainless steel to the glass was defined as time 0. Aliquots (3 mL) were taken from each sample at 0, 1, 7, 14, and 24 days and tested for PS80 degradation by LC-MS.
NMR/EPR Radical Trapping Experiments
Radical trapping experiments using NMR and EPR were conducted to determine the types of free radicals present in each sample because of exposure to temperature, light, or stainless steel. Aliquots of the selected samples from the various experiments conducted were spiked with either 4-hydroxy-5,5-dimethyl-2-trifluoromethylpyrroline-1-oxide (FDMPO, 5 mM) or 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO, 6.4 mM) and analyzed using either NMR or EPR, respectively, to determine the types of radicals formed in the samples.
Results and Discussion
Impact of Temperature
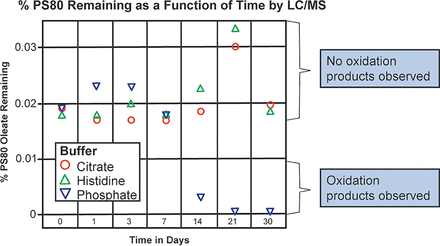
The impact of temperature on PS80 oxidation in the histidine, the citrate, and the phosphate buffers is presented in Figure 1. As demonstrated in this figure, there was no loss of PS80 or formation of PS80 oxidation products in any of the buffers studied for the first 7 days of incubation at 50°C in glass. Loss of PS80 and the presence of PS80 oxidation products appeared within 14 days in the phosphate buffer. However, PS80 appeared to be stable in both the citrate and the histidine buffers for up to 30 days, suggesting that the free radicals are either not formed by temperature in these buffer systems or that they are formed and quenched by these buffer systems.
Effect of temperature (50°C) on PS80 oxidation in the histidine, the citrate, and the phosphate buffers as a function of time. All buffers were 10 mM in concentration with 0.02% PS80 and were stored in glass.
Impact of Light
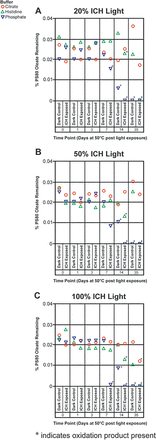
The impact of ICH light on PS80 oxidation in the histidine, the citrate, and the phosphate buffers is presented in Figure 2. Over the course of the first three days, no PS80 oxidation was observed in any of the samples. For all the phosphate buffer samples exposed to light, PS80 oxidation products were observed within 7 days and complete loss of PS80 occurred within 14 days. The dark control from the phosphate buffer also showed some PS80 oxidation within 14 days, which is consistent with the results from the impact of temperature with this buffer system (Figure 1). The histidine buffer samples exposed to light also showed PS80 oxidation within 14 days at all light conditions, while the dark controls had intact PS80. Clearly, the oxidation occurring in the phosphate buffer is likely because of both temperature and light whereas the oxidation occurring in the histidine buffer is only because of light. The overall metals load in these buffers were comparable by inductively coupled plasma mass spectrometry (data not shown). One possible explanation (16) for the oxidation of PS80 in the phosphate buffer because of both temperature and light is that phosphate has a strong tendency to adsorb iron oxides from surfaces as it enhances the rate of Fe(II) oxidation—transformation of Fe(II) to Fe(III)—with subsequent formation of iron oxides. Overall, the three different ICH light conditions rendered consistent results with greater degradation occurring with 100% ICH Q1B light than with 50% ICH Q1B light conditions, and greater degradation occurring with 50% ICH Q1B light than with 20% ICH Q1B light conditions. The citrate buffer samples exposed to 100% ICH Q1B light showed some PS80 oxidation after 35 days of incubation, whereas the samples exposed to 50% and 20% ICH Q1B did not show any PS80 degradation products. One likely explanation for this observation is that 100% ICH Q1B light generates more free radicals than the citrate can quench, leading to oxidation.
Effect of 20% (A), 50% (B), and 100% (C) ICH Q1B light on PS80 oxidation in the histidine, the citrate, and the phosphate buffers. Buffers were stored at 50°C after light exposure and PS80 oxidation was monitored as a function of time, with T = 0 defined as the time after light exposure before storage at 50°C. All buffers were 10 mM in concentration with an initial 0.02% PS80 concentration. Dark controls for each buffer were treated identically to the light-exposed samples but were covered in foil before exposure to light.
Impact of Contact with Stainless Steel (316)
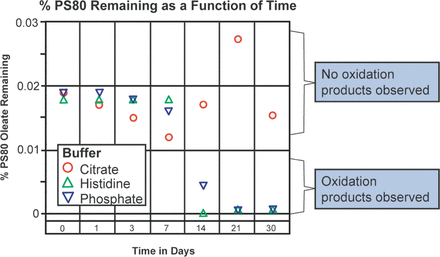
The effect of contact of the three buffer systems with 316 stainless steel at 50°C is presented in Figure 3. As was the case with the temperature study, no degradation of PS80 was observed for the first 7 days with any of the buffer systems. However, PS80 oxidation products were observed by LC-MS within 14 days in both the histidine and the phosphate buffers. No loss or oxidation of PS80 was observed in the citrate buffer system, consistent with our earlier report. This is likely because of the weak chelating and/or radical scavenger properties of citrate.
Effect of stainless steel at 50°C on PS80 oxidation in the histidine, the citrate, and the phosphate buffers as a function of time. All buffers were 10 mM in concentration with 0.02% PS80 and were stored in glass.
Mechanistic Understanding of the Function of Citrate and EDTA in Preventing/Minimizing PS80 Oxidation:
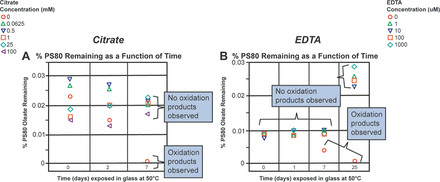
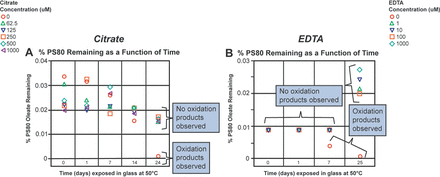
After observing the stability of PS80 in the citrate buffer when exposed to stainless steel at 50°C, it was of interest to study the ability of citrate to prevent PS80 oxidation in other buffer systems where PS80 oxidation was observed upon contact with stainless steel. The histidine buffer was chosen for this study. In our previous study, we showed that 100 ppm EDTA (342 µM) prevented PS80 oxidation in a histidine buffer (11). Although EDTA is more frequently used than citrate as a chelator in formulations, it presents challenges because of the adverse effects that have been observed with it in vivo (17⇓⇓–20). In this study, citrate and EDTA were compared for their ability to quench the potential radicals formed in the histidine buffer. The results are presented in Figure 4. As shown in Figure 4A, low levels of citrate (∼62.5 µM) prevented PS80 oxidation in the histidine buffer for up to 7 days, whereas the control sample without citrate showed complete loss of PS80 because of oxidation upon exposure to stainless steel. Because EDTA is a much stronger chelating agent than citrate, it was assessed at lower concentrations, ranging from 0 to 1000 µM. As shown in Figure 4B, 1 µM EDTA was able to prevent PS80 oxidation in the histidine buffer for up to 25 days at 50°C, whereas the control sample with no EDTA started showing oxidation within 7 days and showed complete PS80 oxidation within 25 days. The data presented in Figure 4B displayed noticeable variability throughout the duration of the study. The variability in the data set might be attributable to the fact that samples through 7 days were analyzed in a single run, whereas the 25-day samples were analyzed separately. However, PS80 oxidation started to occur on day 7 with complete loss at day 25, indicating oxidation was still occurring in the absence of EDTA. This variability could have been minimized if an internal standard for PS80 had been available to use. The internal standards typically normalize the results, leading to minimal data variability. Thus, it is important to look at the chromatograms for intact PS80 and for the presence of oxidation products to determine the PS80 degradation in these buffers.
After observing the ability of citrate and EDTA to prevent PS80 oxidation in the histidine buffer, understanding the mechanisms behind these observations was of interest. Our hypothesis was that either citrate and EDTA are chelating the metal ions and preventing the free radicals from being formed or that they are quenching the free radicals formed. A study, similar to the one above, was performed, in which the histidine buffer containing 0.02% PS80 (w/v) was incubated in stainless-steel containers for up to 24 h at 50°C. The studies conducted earlier by this lab and in our earlier publication confirm that brief exposure of the histidine buffers with PS80 to stainless steel is sufficient to induce PS80 oxidation. Hence, a conservative 24 h incubation time was chosen to ensure that free radicals were formed. As described in the experimental section, buffer solutions were spiked with either citrate or EDTA after 24 h incubation in stainless steel. As seen in Figure 5A, the PS80 in any sample containing 62.5 µM of citrate did not appear to undergo oxidation, whereas the PS80 in the control sample (0 μM citrate) appeared to be completely oxidized by day 24 at 50°C, as confirmed by the presence of oxidation products. Figure 5B shows similar results for EDTA, in which samples spiked with 1 µM of EDTA did not show any PS80 oxidation, whereas the PS80 in the control sample (0 µM EDTA) appeared to be completely oxidized by day 25, as confirmed by the presence of PS80 oxidation products with LC-MS. The lack of oxidation observed in the presence of citrate or EDTA suggests citrate and EDTA act by quenching the formed free radicals. Furthermore, the assumption that free radicals are formed during the first 24 h of incubation in stainless steel is validated from the above temperature study, in which the results indicate no oxidation of PS80 in the histidine buffer for up to 30 days when not exposed to stainless steel, but oxidation of PS80 in the absence of citrate or EDTA does occur when exposed to stainless steel for 24 h. Thus, the 24 h stainless-steel exposure at 50°C may have caused the formation of free radicals that led to PS80 oxidation in the control samples.
Ability of citrate and EDTA to prevent PS80 oxidation in the histidine buffer. (A) Different concentrations of citrate (100 to 0 mM) were spiked into the 10 mM histidine buffer with 0.02% PS80 and then PS80 oxidation was monitored as a function of time. (B) Different concentrations of EDTA (1000 to 0 µM) were spiked into the 10 mM histidine buffer with 0.02% PS80 and then PS80 oxidation was monitored as a function of time.
Ability of citrate and EDTA to prevent PS80 oxidation in the histidine buffer after exposure to stainless steel at 50°C for 24 h. (A) Different concentrations of citrate (100 to 0 mM) were spiked into the 10 mM histidine buffer with 0.02% PS80 and then PS80 oxidation was monitored as a function of time. (B) Different concentrations of EDTA (1000 to 0 µM) were spiked into the 10 mM histidine buffer with 0.02%PS80 and then PS80 oxidation was monitored as a function of time.
Iron Spiking in Addition to Stainless-Steel Contact Study:
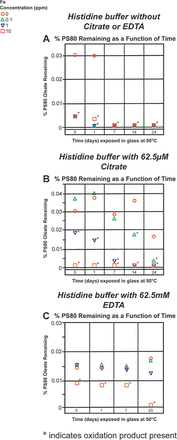
Finally, the ability of citrate and EDTA to prevent PS80 oxidation in the histidine buffer with added iron that was exposed to stainless steel was investigated. The pharmaceutical industry predominantly uses 316 stainless steel for the manufacture of parenteral products. The reason for conducting the spiking studies with iron is because in addition to nickel, chromium, molybdenum, and so forth, the 316 stainless steel also contains ∼75% iron, which seems to play a critical role in Fenton chemistry (21⇓⇓–24). As documented, Fenton chemistry is one of the major degradation routes for the oxidation of PS80. Also, as many excipients used in formulations come with trace levels of metals, such as iron, it is not uncommon to have trace amounts of these contaminants present (15). Trace levels of metal contaminants can cause PS80 oxidation; therefore, it was of interest to see if citrate and EDTA can prevent PS80 oxidation in the histidine buffer with Fe2+ added in the formulation. Different levels of Fe2+, from zero to 10 ppm, were spiked into the histidine buffer containing PS80 that was placed in contact with stainless steel. The concentration of 62.5 µM for citrate was chosen, as it was the lowest concentration evaluated in the previous studies and was found to be just as effective as 1000 µM of citrate. Although EDTA is a stronger chelator than citrate, an equal concentration was used so that a direct comparison could be made between citrate and EDTA. The spiked buffer samples were exposed to stainless steel for 24 h at 50°C and then transferred to glass for further incubation at 50°C. Figure 6A shows the effect of added iron on PS80 in the histidine buffer without citrate or EDTA. In the absence of citrate, EDTA, or Fe2+, PS80 is almost completely oxidized by 7 days after stainless-steel exposure. However, our previous study showed complete PS80 oxidation over a period of 24–25 days. One possible explanation for this difference could be the differences that may have occurred in the passivation of the stainless-steel surface. With the added Fe2+, the process of PS80 oxidation occurs much faster with iron acting as a catalyzer. Figure 6B demonstrates the effect when 62.5 µM citrate is added to the formulation. Based on previous results, citrate prevents PS80 oxidation in the histidine buffer without endogenous Fe2+. However, when 10 ppm of Fe2+ was added, the oxidation of PS80 is complete within 24 h of exposure to stainless steel. When 1 ppm Fe2+ was added to the buffer solution, the PS80 oxidation is much slower, leading to complete oxidation within 7 days. Likewise, when 0.1 ppm Fe2+ was added to the histidine buffer containing PS80, oxidation products were not observed until day 14. However, complete loss of intact PS80 was observed by day 24.
Ability of citrate and EDTA to prevent PS80 oxidation in the histidine buffer in the presence of excess iron (10, 1, and 0.1ppm Fe2+) and stainless-steel exposure. (A) Monitoring the intact PS80 in the 10 mM histidine buffer without citrate or EDTA, with different levels of excess Fe2+. (B) Monitoring the intact PS80 in the 10 mM histidine buffer with 62.5 µM citrate, with different levels of excess Fe2+. (C) Monitoring the intact PS80 in the 10 mM histidine buffer with 62.5 µM EDTA, with different levels of excess Fe2+.
Figure 6C shows the effect of 62.5 µM EDTA on PS80 oxidation in the histidine buffer with added iron. Because the threshold for EDTA to chelate metals is higher than that for citrate, EDTA is expected to be more effective in delaying or preventing PS80 oxidation (11). Based on the results, 62.5 µM EDTA can prevent PS80 oxidation in the histidine buffer for up to 25 days even in the presence of 0.1 and 1 ppm of added Fe2+. However, the 10 ppm Fe2+ concentration was able to completely saturate the EDTA, and oxidation was observed at time 0 with complete oxidation by day 25. The results from the EDTA study can be better understood by examining the stoichiometry of the samples. The 10 ppm, 1 ppm, and 0.1 ppm Fe2+ samples contained Fe2+ at a concentration of 180, 18, and 1.8 µM, respectively. With an EDTA concentration of 62.5 µM, it is expected that up to 62.5 µM Fe2+ can be chelated. This explains the lack of oxidation observed in the 18 µM (1 ppm) and the 1.8 µM (0.1 ppm) added Fe2+ samples and the occurrence of oxidation in the 180 µM (10 ppm) Fe2+ sample. One would expect the same explanation to be valid for the 62.5 µM citrate sample, but this does not appear to be the case likely because of the difference in the binding constants for the Fe2+–EDTA and Fe2+–citrate complexes. The binding constant for iron to citrate appears to be about threefold weaker than the binding constant for iron to EDTA, which explains why oxidation was observed in the citrate-containing buffer despite the favorable stoichiometry.
NMR and EPR Radical Trapping Studies
To determine the identity of the free radicals formed under the various conditions tested, NMR and EPR spin-trapping experiments were performed. The results were inconclusive (data not shown).
Conclusion
While it was previously thought that temperature is an initiator of PS80 oxidation in different buffer systems, this study demonstrated that temperature up to 50°C does not initiate PS80 oxidation in either the histidine or the citrate buffer systems but may be responsible for the PS80 oxidation in the phosphate buffer. As with temperature, light has also been considered to be an initiator of PS80 oxidation in different buffer systems and that was proven to be true in this study. Whereas PS80 in the phosphate buffer seemed to be the most sensitive to light, PS80 in both the histidine and the citrate buffers also underwent oxidation although at a much lower level; the PS80 in the histidine buffer underwent oxidation at a more rapid pace compared to the PS80 in the citrate buffer. Finally, the ability of citrate and EDTA to act not only as chelators but also as radical quenchers/scavengers in the presence of stainless steel and added metals such as Fe2+ and to prevent or delay PS80 oxidation in the histidine buffer was also demonstrated. Although no radicals were trapped or detected by either NMR or EPR, it is understood that radicals should have been present in the system at some point in time because PS80 oxidation products were observed and PS80 oxidation is well-known to be a radical-mediated phenomenon.
Conflict of Interest Declaration
The authors declare that they have no competing interests related to this article.
Acknowledgements
The authors would like the thank Dr. Michael Zhuo Wang for allowing his graduate student, Laura Doyle (Drbohlav), to come to Eli Lilly and Company to perform these studies. In addition, the authors would like to acknowledge Justin Douglas from the NMR Core Lab at the University of Kansas for his time in running the EPR experiments. The authors would also like to thank Natarajan Rajagopalan for providing technical guidance and thorough review of the manuscript.
- © PDA, Inc. 2019