Abstract
The pharmaceutical industry has been confronted with new and complex challenges, particularly with regard to the aseptic filling of parenterals, including monoclonal antibodies and ophthalmologic drugs designed for intravitreal injections, which often require fill volumes <200 µL. In addition to intravitreal administration, microliter doses may be required for applications using highly concentrated formulations and cell and gene therapies. Many of these therapies have either a narrow or unknown therapeutic window, requiring a high degree of accuracy and precision for the filling system. This study aimed to investigate the applicability of a linear peristaltic pump as a novel and innovative filling system for the low-volume filling of parenterals, compared with the state-of-the-art filling systems that are currently used during pharmaceutical production. We characterized the working principle of the pump and evaluated its accuracy for a target fill volume of 50 µL. Our results demonstrated that the linear peristaltic pump can be used for fill volumes ranging from 12 to 420 µL. A deeper investigation was performed with the fill volume of 50 µL, because it represents a typical clinical dose of an intravitreal application. The filling accuracy was stable over an 8 h operation time, with a standard deviation of +/−4.4%. We conclude that this technology may allow the pharmaceutical industry to overcome challenges associated with the reliable filling of volumes <1 mL during aseptic filling. This technology has the potential to change aseptic filling methods by broadening the range of potential fill volumes while maintaining accuracy and precision, even when performing microliter fills.
Introduction
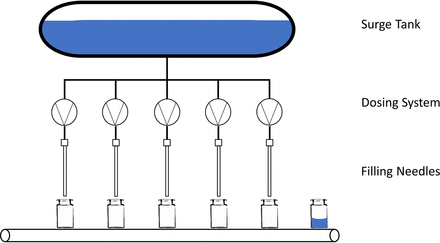
The potency of new drugs has resulted in the use of smaller volumes for therapeutic applications (1). Additionally, many pharmaceutical companies focus their portfolio on intraocular injections. Therefore, the drug manufacturers must adapt to changes and challenges encountered with the filling of small volumes during the completely aseptic process. Existing commercial state-of-the-art filling lines were typically designed for the high-throughput production of hundreds of thousands of vials or syringes during every shift. Each aseptic filling suite supports different unit operations with distinct purposes that contribute to the aseptic manufacturing process, such as sterile filtration, filling into the primary container, container closure, and sealing. Figure 1 provides a schematic overview of an aseptic filling process.
Schematic view of a filling process, with five filling systems and filling needles. Single-use tubing connects the central filling systems to the surge tank, which contains a reservoir of the drug product solution (in blue), and to the filling needles.
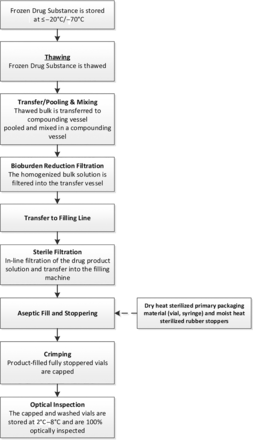
The current trend toward increasingly personalized and highly potent therapies has resulted in the need to develop microliter aseptic fill-finish systems, to deliver low fill volumes, such as the 50 µL volumes required for intravitreal injections, with the high level of accuracy and precision that characterize current filling systems. A flow diagram describing a representative aseptic drug product manufacturing process is shown in Figure 2.
Example of an aseptic manufacturing process. Flowchart showing the steps followed during a representative aseptic manufacturing process.
The United States Pharmacopeia (USP) General Chapter <1151> describes the acceptance criterion that the average contents of all tested samples must not be <100% of the labeled amount (2). It is therefore common industry practice to have a small overage in vials to allow for the correct withdrawable volume using a syringe for administration. The volume in the vial required to obtain the appropriate withdrawable volume is determined in laboratory studies. The industry as a whole is concerned that the process of overfilling could lead to the repeated use of a single vial or the pooling of leftover drug products from multiple vials to obtain a single dose. Additionally, there is the risk of users misusing the product and injecting the entire contents rather than dosing from a higher volume. These practices would expose patients to adverse events caused by microbial contaminations and overdoses (2⇓⇓–5).
The current method of choice for the administration of a drug representing a 50 µL volume to a patient is the use of prefilled syringes (PFSs), which facilitates the application of the target dose from a larger fill volume (e.g., 50 µL out of 0.5 mL), based on dose marks on the PFS. Intravitreal administrations using PFSs resulted in a 50% reduction in infectious endophthalmitis cases when compared with administrations from a vial (6). However, PFSs can have internal diameter variations of ±0.1 mm, resulting in delivered volume variations of up to ±2.2 µL. The process used to apply the external dose mark on the syringe can yield a dose mark tolerance of ±0.25 mm, which can result in variations of up to ±4.3 µL in the delivered volume (7). These two factors introduce the potential for inaccurate delivered doses before considering any operator-related inaccuracies or imprecisions. A human factor study revealed that the average dose administered when aiming for a 50 µL target dose by dosing from a 500 µL PFS was 56.2 µL (12% error) (7). Because the ratio between the syringe volume and the intended application volume plays an important role in determining both accuracy and precision, smaller syringe sizes and dosing systems capable of filling small volumes are necessary (8⇓–10).
Filling Systems
Various filling systems are currently available on the market and are currently in use during pharmaceutical production, and each system has advantages and disadvantages. The following sections will provide an overview of the most commonly used filling systems in the pharmaceutical industry and highlight a novel and innovative filling technology, capable of overcoming the challenges and restrictions associated with filling volumes <1 mL.
Table I provides an overview of the four filling systems discussed in this article. Each filling system has strengths and weaknesses that must be considered when choosing a filling system to perform an intended application.
Overview of the Different Dosing System Characteristics
Piston Pump
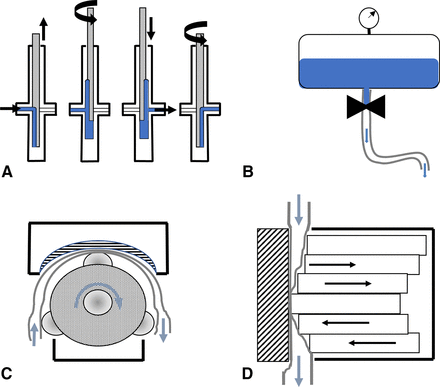
The rotary piston pump (Figure 3A) consists of a cylinder and a “truefit” stainless-steel or ceramic piston. The filling process can be divided into three different steps. First, the uplifting of the piston in the first position creates a pocket of reduced pressure in the filling system, resulting in fluid being drawn into the reservoir. The piston has a one-sided notch in the direction of fluid entry. Second, the piston rotates 180°, placing the notch in the direction of the cylinder outlet. In the final step, the piston moves downwards, delivering the fluid from the cylinder into the filling needle.
Schematic presentations of the investigated pump types. (A) Rotary piston pump. (B) Time-pressure filling system. (C) Radial peristaltic pump. (D) Linear peristaltic pump. Blue arrows indicate the direction of liquid drug product movement. Black arrows indicate the directions of system component movements.
The piston pump is often used specifically for high-precision, aseptic filling because the fill volume can be adjusted in a very precise manner. Every piston pump is limited to a designated fill volume range; therefore, different pump sizes are necessary for different ranges of fill volumes. The piston pump has a high degree of filling accuracy and precision, particularly for low fill volumes (≤1 mL) (11). However, multiple studies have reported elevated subvisible particle counts and a few visible particles in protein formulations filled using piston pumps, leading some to suggest that piston pumps may cause the denaturation and aggregation of delicate biopharmaceutical products sensitive to related product stress (12⇓⇓⇓--16).
Time-Pressure System
In a time-pressure filling system (TPS), the fluid is stored in a surge tank, overlaid with an inert gas (usually nitrogen) that is maintained at constant pressure (Figure 3B). The fluid is distributed by a manifold, through the tubing, to the filling needles. The fill volume is controlled by valves, which are opened for a defined time, the overlaid pressure, and the diameter of the tubing. Product temperature affects filling accuracy (indirectly, via changes in the density and viscosity of the fluid), and, therefore, must be controlled. The TPS is more prone to bubble formation than other systems because of the pressurized surge tank. The overlaid gas pressure increases the concentration of soluble gas in the cooled liquid, and these gases may expand when the liquid moves out of the surge tank, negatively influencing the filling accuracy. This would be especially relevant for small fill volumes such as <1 mL. The machine setup and environmental variabilities, such as pressure fluctuations in the surge tank and temperature changes in the bulk solution, can also influence the accuracy. The TPS requires complicated computer systems and fast actuators to ensure reliable process control and is the most inaccurate of the described filling systems.
Radial Peristaltic Pump
Radial peristaltic pumps (RPPs, Figure 3C) are composed of a rotor, a stator (counterpressure plate), and tubing. The pump acts as the stator, and the three rollers (which are driven by a servomotor mounted on the pump head) act as the rotor. The counterpressure plate is adjustable and can be adapted to the thickness of the tubing. Fluid delivery, in the direction of the filling needle, is achieved by the positive displacement of a liquid column, which moves from a reservoir toward the filling needle. The tubing is squeezed between the rollers and the counterpressure plate by the radial movement of the rollers. After squeezing, the tubing relaxes to its original shape, creating a low-pressure pocket that draws additional fluid into the tubing.
The fill volume is determined by the inner diameter (ID) of the tubing, the tubing elasticity, the number of rollers, the counterpressure, the rotation angle, and the size of the pump head. One advantage associated with RPPs is that the biopharmaceutical solution is only in direct contact with the disposable and single-use tubing, not with the pump itself, which can minimize the required cleaning and sterilization efforts. Multiuse components with direct product contact can be limited to the surge tank and filling needles. Therefore, the tubing material must be suitable for aseptic fill-finish processes, able to be cleaned and sterilized under conditions of 121°C and 2 bar pressure. Additionally, leachables, extractables, and particle shedding into the fluid must be carefully controlled. Recent studies have indicated that the radial movement of the pump can cause particle shedding of the tubing material during aseptic fill-finish processes (17, 18).
Linear Peristaltic Pump
The linear peristaltic pump (Figure 3D) relies on the same physical principle as the RPP (positive displacement) (19). Because of the subsequent squeezing of the tubing, the forward movement of a fluid column is achieved. The linear peristaltic pump consists of multiple piezo actuators that act in line. The actuators squeeze the tubing through synchronized orthogonal displacement, resulting in a sine wave. Initially, each filling cycle actuator is displaced by 100%, resulting in a phase shift of 90° relative to the base sine wave, which prevents dripping and air entry. Each piezo can be displaced between 0 and 1000 µm. The phase shift of each actuator is fixed 60° relative to the phase of the previous actuator, and the displacement of all six piezos is defined as one cycle. The fill volume is directly controlled by the number of cycles, the number of displaced actuators, the actuator sizes, the precompression of the tubing (by the counterpressure plate), and the elasticity of the tubing. Because of the orthogonal movement and the lack of tangential force vectors, the tubing inner surfaces could potentially experience reduced movement relative to the tubing in the RPP. This would result in reduced material strain and reduced particle shedding.
In addition to the linear peristaltic fill mode, the filling system can also be operated in a time-pressure mode. Within this mode, two piezo actuators in the filling system act as a valve, and the fill volume is controlled comparable to a “standard” TPS, as described previously. In this study, the time-pressure mode was not used; however, it may play an important role in future development, particularly when extending the fill volume from the microliter range to the higher milliliter range.
Aim of the Study
Aseptic fill-finish facilities traditionally use the piston pump–mostly for small molecule and compatible large molecule products–because of its high degree of filling accuracy and precision. However, the trend has shifted in recent years toward RPPs. RPPs generate less product stress for biopharmaceutical drug products and limit the number of product-contacting surfaces (14, 20). The major concern for RPPs is the filling accuracy, particularly when using very small fill volumes <200 µL (11).
To close the gap between the highly accurate but product-straining piston pump and the less accurate but gentler RPP, a linear peristaltic pump prototype was constructed, and the feasibility of its application during the low-volume filling of parenterals was examined in preliminary experiments. Our work focused on the further development of the optimal control parameters for accurate and precise filling. Therefore, we designed a study to monitor a target fill volume of 50 µL, for up to 8 h, which was representative of a typical manufacturing shift. Additionally, we investigated the fill volume range, using two different types of platinum-cured silicone tubing, to frame the scope of the application. As a final step, we investigated whether a potential reduction in the sizes of the piezo actuators would affect the accuracy of the system, to lay the groundwork for future prototype evolutions.
Materials and Methods
All filling accuracy experiments were performed using water or a highly concentrated glycerol-water solution (glycerol-water 60% v/v and 0.02% polysorbate 20). The glycerol-water solution had a measured viscosity of 16.49 cP, which was considered to be the worst-case scenario for standard protein formulation viscosities. For all data analyses, the mass density of the distilled water (δH2O) was assumed to be 1 g⋅cm−3, and the mass density of the glycerol-water solution was measured as 1.1538 g⋅cm−3 at 20 °C. The gravimetric measurement results were directly converted from mg into µl, according to density.
Filling System
The linear peristaltic pump is a patent-granted technology. The pump consists of multiple piezo actuators (P-602 PiezoMove, PI, Karlsruhe, Germany) in a stainless-steel housing and was operated together with platinum-cured silicone tubing (Flexicon Accusil), with IDs of 0.8 mm and 1.6 mm. The wall-strength values of both tubings were 1.6 mm, and the tubings were chosen after a preliminary study that examined the effects of compression and restoration forces on the tubing shape. The tubing was connected to a surge tank on one end and to a low-volume filling needle on the other end. The filling needle had an ID of 0.6 mm and a length of 150 mm. Both the surge tank and the filling needle were made of 316 L stainless steel. For drug products that are sensitive to oxidation, the filling needle has the ability for simultaneously inert gassing with nitrogen. The working principle underlying the pump and the volume control is described, in detail, in the patent (19).
Filling Accuracy Target
The strategy for this technology development was to minimize the filling process variability. We therefore abstained from defining a fill volume acceptance criterion. The overarching aim was to create a drug product presentation in the form of a PFS that does not need dosing based on a dose mark. It would be a great patient benefit if the dose variability introduced by the operator and additional handling steps could be eliminated. For comparison with typical performance data for filling accuracy in the small-volume range, we present the data with a typically process consistency window of ± 10% in the figures.
Filling Accuracy Readout
The filling accuracy and precision were determined by gravimetric measurements. Therefore, a Mettler Toledo balance (XPE105) was used with gravimetric software (GraviDrop, BiofluidiX GmbH, Freiburg, Germany) during all dispensing experiments.
Results
Our results provide an overview about the construction of the linear peristaltic pump as well as its applicability for the aseptic filling of low fill volumes.
Linear Peristaltic Pump: Construction
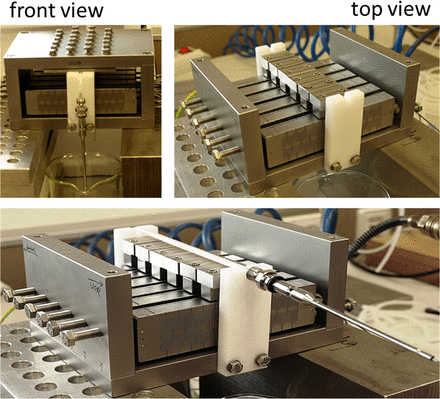
The development and construction of the linear peristaltic pump were described, in detail, in the patent (19). Figure 4 shows an overview of the pump. The pump was constructed from stainless steel and consists of 6 piezo actuators and a counterpressure plate. The fluid was delivered toward the filling needle by positive displacement through a silicone tube, which was fixed between the actuators and the counterpressure plate.
Overview of the linear peristaltic pump construction. The upper left image shows a front view of the pump, with the assembled counterpressure plate, whereas the upper right image shows a top view of the pump, without the counterpressure plate. The bottom image shows how the tubing (black arrow) is placed on top of the actuators and connected to the filling needle.
Linear Peristaltic Pump: Parameter Settings
The fill volume control was determined by multiple variables, using both fill modes (A: linear peristaltic and B: time-pressure). For the linear peristaltic mode, the fill volume was determined by the following variables:
The wavelength of the sine wave
The amplitude of the sine wave
The frequency of the sine wave
The phase shift of the sine wave that controlled the different actuators
The number of actuators being controlled
The tubing geometries
The precompression (offset) of the tubing
The elasticity of the tubing
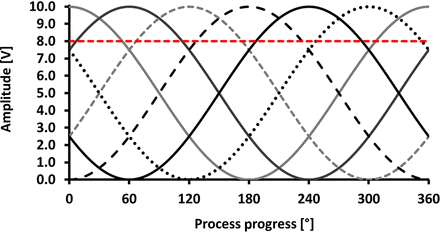
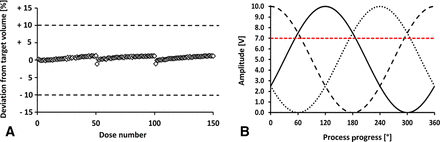
The wavelength of the sine wave and the amplitude represented the preferred control mechanisms, as the tubing properties, the distance between each actuator, and the number of actuators remained constant. Figure 5 shows an overview of the positive displacement for all six actuators. The position 0° was the starting point of the filling process. Prior to any filling step, the tubing must be manually adjusted and controlled, especially if it becomes tight after reaching a certain displacement of the actuators. The chosen displacement for a full closure of the tubing was set to an offset of 8 V (80% displacement). This setting was necessary because the counterpressure plate was slightly variable, and the tubing must be able to be sealed at any of the six actuator positions to prevent uncontrolled liquid flow. Figure 5 shows that at least one actuator is sufficiently displaced to seal the tubing at any given time during the filling. In our model, actuator 1 entered the sine cycle at full amplitude, which prevents air entrainment in the system or the leakage of the level tank. Actuators 2–6 were shifted by 60° relative to the phase of the previous actuator.
Visualization of the parameter settings used for a 50 µL target fill volume. The process progress is measured in degrees, with 360° representing a full filling cycle. The amplitude is measured in volts, with 10 V representing the full amplitude of positive displacement. The red dotted line indicates the actuator position at which the tubing will be sealed completely.
Linear Peristaltic Pump: Filling Accuracy
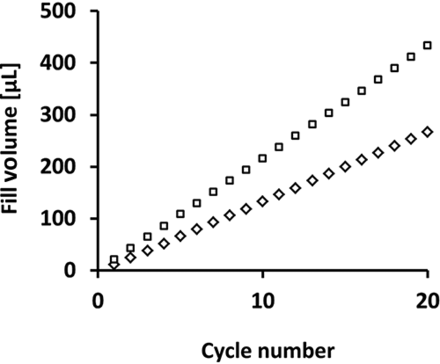
During preliminary tests, the volume range of the linear peristaltic pump was tested using two different silicone tubing diameters (ID = 0.8 mm and ID = 1.6 mm). Figure 6 shows the volume coverage of the pump for each tubing diameter, with increasing numbers of sine wave cycles.
Fill volume range of the linear peristaltic pump. Diamonds indicate fill volumes when using silicone tubing with an inner diameter of 0.8 mm with a maximum deviation of 0.64%. White squares reflect the fill volumes when using silicone tubing with an inner diameter of 1.6 mm with a maximum deviation of 0.43%. The data points shown reflect the means of three independent experiments.
The increase in volume was proportional to the increase in the number of sine wave cycles, as expected, and a volume range from 12 to 430 µL was achievable when using as many as 20 sine wave cycles. Although higher fill volumes are possible when using the linear peristaltic mode, the time required for a single fill when using >20 sine wave cycles would be too long for commercial filling processes. Therefore, the time-pressure mode should be used for fill volumes >500 µL.
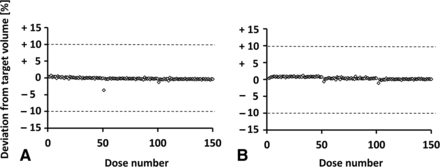
To achieve a target fill volume of 50 µL, a platinum-cured, silicone tubing with an ID of 0.8 mm and a wall strength of 1.6 mm was used, and 24 actuators were displaced, in total, equal to 4 complete sine wave cycles for each of the six actuators. The frequency was set to 30 Hz, resulting in a total fill time of 133 ms. Although various frequencies were examined during preliminary experiments (data not shown), no improvements for the process were observed when using different frequencies. When the frequency was <15 Hz, no fluid transport was possible, and this effect was especially noticeable for high-viscosity solutions because of the increased mass inertia. At frequencies >40 Hz, the tubing began to vibrate, and no fluid transport was possible in a controlled manner. Figure 7 shows an overview of the filling accuracy of 3 × 50 µL single-filling steps (A: water; B: glycerol-water solution, cP 16.49). The highly viscous model solution was chosen to investigate the effects of higher viscosities on the filling accuracy and consistency of the linear peristaltic pump.
Filling accuracy for 3 × 50 single filling events. (A) The filling accuracy for purified water (viscosity = 1 cP). (B) The filling accuracy for a high-viscosity glycerol–water solution (16.49 cP). Dotted lines indicate the accepted deviation range of ±10%.
After each set of 50 fills, a 10 min process interruption was simulated. The fill volume was collected in a plastic beaker on a high-precision gravimetric balance (Mettler Toledo XPE105), and the volume was calculated based on the mass and density of the solution.
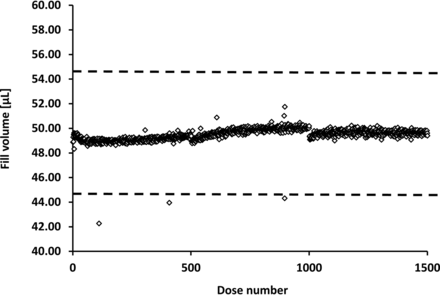
Both tests showed that the linear peristaltic pump was capable of filling fluids within a viscosity range of 1–16.49 cP at targeted volumes of 50 µL. To further investigate the filling accuracy and consistency over time, a long-term filling test was performed for >8 h, which represents the length of a typical shift during commercial production, during which 1500 single filling steps were performed (Figure 8). The filling speed was consciously reduced to allow for the optimal settling time during gravimetric measurements.
Long-term filling accuracy for water. Repeated single fills, with targeted fill volumes of 50 µL, were performed for >8 h (representing 1500 individual filling events). The dotted lines indicate the acceptable upper and lower 10% deviation limits for low fill volumes. A total of three individual filling events were identified as being outside of the acceptable range because of insufficient control of the environmental surroundings (e.g., air-pressure changes because of door openings).
The long-term filling test confirmed the preliminary accuracy and consistency data, over a time period of >8 h, without any feedback loop (industry standards for aseptic filling). Additionally, no tubing fatigue was observed, which would typically be observed with an RPP system.
Linear Peristaltic Pump: Filling Accuracy After Actuator Reduction
The linear peristaltic pump remains in the prototype stage and requires further improvements before the full system can be implemented on a commercial filling line. To achieve the optimal filling accuracy, the dosing element should be co-located with the filling needle to prevent any pressure drops over the tubing length, which could lead to worse filling accuracy. Currently, the pump weighs approximately 20 kg, which is not suitable for fitting directly onto the filling needle. Therefore, additional tests to investigate potential methods for size and weight reductions in the pump prototype were performed (Figure 9). The most promising improvement for the future commercial implementation of this pump would be the reduction of the piezo actuators, which would minimize both the size and the weight of the pump.
Filling accuracy after reducing the actuators by 50%. (A) The filling accuracy of 3 × 50 single filling events at a target fill volume of 48 µL, when only actuators 4–6 were in operation, using 13 sine wave cycles. The dotted lines indicate the acceptable deviation range of ±10%. (B) Visualization of the parameter settings. A full filling cycle is represented as 360°, and the amplitude indicates the displacement of the actuators. The tubing was sealed after an actuator displacement of 7 V (70%, red line). The filling speed was set to 20 s/filling event, at 30 Hz (433 ms).
A test was performed using only three operational actuators, to investigate whether the filling accuracy and consistency were affected by a reduction in the number of actuators. Three represents the minimum number of actuators required for fluid transport, as two would merely move the fluid back and forth. The testing parameters used were identical to those used in the previously described filling accuracy tests. The amplitude for the state during which the tubing remained fully sealed was lowered to 7 V to ensure that at least one closed position was recorded for each state of the fill.
Discussion
The filling accuracy of the linear peristaltic pump showed a maximum relative standard deviation of 0.65% within a fill volume range of 12–450 µL. The filling accuracy studies were performed with set parameters, which are shown in Figure 5. The piezo actuators were displaced by continuous movement, following the shape of a sine wave. Therefore, the fill volume of the pump was not limited to one fill cycle (displacement of piezos 1–6) because the wave continued at actuator 1 after reaching the last actuator (in this case, actuator 6). Because of this continuous movement, the fill volume could be adjusted in a very precise and accurate manner. Figure 4 shows the fill volume ranges for both tubing sizes used in the study.
We successfully demonstrated that linear peristalsis could cover a fill volume range from 12 to 420 µL, with a linear relationship between the number of sine wave cycles and the total fill volume. This linear behavior confirmed that the fill volume was directly dependent on the number of piezo movement cycles. The linear peristaltic pump requires further improvements to prepare it for use in a good manufacturing practice (GMP) environment. Currently, the tubing seal must be adjusted by hand, depending on the piezo offset, and this process was necessary to prevent any deviations caused by small disparities in the tubing material or the piezo mounting, particularly because the prototype was manually built. Future processes should be fully automated, such as by measuring the airflow through the needle at constant pressure and exactly adjusting the distance required for full tubing closure.
Another advantage of the linear peristaltic pump is that the whole system can be established before the regular clean-in-place (CIP)/sterilize-in-place (SIP) processes, which occurs before aseptic filling. Usually, components must be placed after CIP/SIP, through manual manipulation, by gloved workers in a sterile environment. Therefore, the linear peristaltic pump could potentially minimize human interference with the aseptic system.
To enhance the applicability of the linear peristaltic pump for an even broader fill volume range, the fill volume range can be divided into submilliliter and milliliter ranges. For drug products requiring submilliliter fills, the pump should be operated in linear peristaltic mode. For fill volumes in the milliliter-range, the system can be switched from linear peristaltic mode to time-pressure filling, without requiring major adjustments to the pump system. The only difference would be the pressure control of the surge tank. This two-mode operation system would enable pharmaceutical companies to cover the complete product portfolio of drug fill requirements with a single filling system. The present study focused on the accuracy and precision of the linear peristaltic mode for targeted fill volumes of 50 µL, which is the typical application dose for intravitreal injections.
As shown in Figure 6, the fill volumes of three sets of 50 fills, using 50-µL target volumes, remained stable and met the process consistency window of ±10%. Figure 7 shows that the first fill volume after the 10 min process interruption was slightly lower, before reaching the initial volume within the next 2–3 fills.
This effect may be caused by the following issues. (1) During the 10 min break, the tubing may be compressed at one position, resulting in the tubing being flattened and regaining its shape after the filling process started again. This effect has been demonstrated in RPP studies, which showed that some tubing did not regain its original shape, even after one week (11). (2) Depending on the fluid properties and the needle material, liquid level variations may occur at the end of the filling process because of the rapid suck-back of liquid into the needle tip. Shieu et al. showed that reductions in liquid pressure, using custom adjustments, could minimize this effect (21). (3) A slight amount of liquid may evaporate from the needle during the 10 min break, resulting in this volume being absent from the first fill following process interruption. In another experiment, the filling accuracy was tested for 8 h, which was equivalent to a standard shift during commercial production (Figure 8). The filling accuracy remained stable for the entire 8 h filling period without any major interruptions. The only process interruptions occurred after every 500 filling events, when the reservoir on the gravimetric balance was emptied. The maximum deviation measured over 8 h of continuous dosing was 4.4%, which was well-below the 10% limit. Three clear outliers were identified, for which the volume was underfilled.
A follow-up experiment, in which only the background was monitored for 8 h, revealed a comparable number of outliers over time (data not shown). The gravimetric balance was not installed on a massive weighing table, which is normally decoupled from the environment. Therefore, some background noise, caused by air turbulence and vibrations, may have influenced the gravimetric measurement. This effect is known to occur during gravimetric in-process control during commercial manufacturing and has been investigated, in detail, in another study (22).
Another effect that could negatively influence filling accuracy is the use of connective tubing between the pump and the filling needle, which is a common practice during commercial manufacturing. Previous studies demonstrated decreased filling accuracy over the length of the tubing used to connect the filling system with the filling needle. Poor filling accuracy occurred because of fluctuating fluid column pressures between the pump and the filling needle. The Hagen–Poiseuille law describes the dependency of pressure loss on the tubing length. For smaller fill volumes, this effect can become especially relevant because any small deviations can lead to under- or overfilled drug products that would be rejected by the in-process control system. Therefore, the filling needle was connected directly to the pump for all filling accuracy studies. To achieve a degree of co-localization between the filling system (point of dose = PoD) and the filling needle (point of fill = PoF) that would be suitable for commercial manufacturing, the pump must be revised and miniaturized. Currently, the actual size and weight of the pump make it impossible to mount a needle-attached pump on a movable filling bar.
All filling accuracy experiments were performed without any applied suck-back mechanism. As discussed previously, a weak suck-back effect may have occurred, triggered by fluid dynamics and material properties, but not actively controlled by the filling system. During suck-back, a defined volume of the fluid is withdrawn from the needle tip, to prevent the tip from clogging. The suck-back mechanism of a filling system becomes increasingly important with highly concentrated and low-volume protein formulations. Because of the high proportion of solid fractions in liquid protein formulations, they tend to dry faster than standard formulations (21, 23⇓–25). In addition to the prevention of potential needle clogging, the suck-back mechanism also minimizes contact between the fluid and air, which protects the product from exposure to interfaces that can lead to protein particle formation (20, 26, 27). For future implementations, a controllable suck-back mechanism should be developed.
In a final experiment, the possibility of reducing the sizes of the piezo actuators was investigated to determine the potential for the future miniaturization of the pump. The number of active piezo actuators was reduced by 50%, from six to three (Figure 9), and accuracy was examined, using similar procedures as the other accuracy experiments described. The reduction from six actuators to three had no negative influences on filling accuracy or precision.
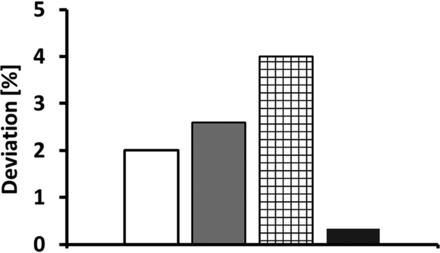
This finding suggests the potential for future pump optimization and miniaturization improvements that may increase the applicability of the pump for commercial manufacturing processes. Finally, the linear peristaltic pump was compared to three state-of-the-art filling systems investigated by Peterson et. al in the fill volume range between 0.03 and 1 mL. Figure 10 shows the deviation from the target fill volume in percentage. It is obvious that among all four filling systems, the linear peristaltic pump performed with the lowest deviation from the target fill volume and can therefore be considered as a valid alternative for aseptic filling, especially in the low volume range <1 mL.
Filling accuracy of the linear peristaltic pump and three commercially available filling systems. The commercially available filling systems (White column: Rotating piston pump; Gray column: Time-pressure filler; Black mesh: Radial peristaltic) showed a deviation from 2% to 4% to a target fill volume of 70 µL. The linear peristaltic pump showed a deviation of only 0.34% to an even lower target fill volume of 46 µL. Data for the commercial filling systems was extracted from: Peterson et. al., Figure 11 (11).
Conclusion
Our study showed that our linear peristaltic pump prototype was able to fill volumes as low as 12 µL, with a maximum fill volume of up to 420 µL. The target fill volume of approximately 50 µL was achieved with a maximum deviation of 0.34% and was maintained within the process consistency window of ±10% during repeated filling for up to 8 h. Because of the two-operation mode potential, the linear peristaltic pump could represent a valid alternative to the state-of-the-art filling systems that are currently in use, by covering the full range of fill volumes required for typical injectables. However, before any routine applications, the pump must be further improved and adapted (e.g., reduction in size and weight).
Conflict of Interest Declaration
The authors declare that they have no competing interests.
- © PDA, Inc. 2021