Abstract
Liquid-in-vial drug products are typically overfilled to meet the label claim volume specification while taking into account losses in the container-closure system and withdrawal device. Any overfill volume setting requires justification. The aim of this study was to estimate the overfill volume required for a liquid drug product in a vial using a prediction model. Glass vials sized from 2R to 20R capacity were filled with sorbitol-based aqueous solutions having a viscosity at 20°C ranging from 1 to 40 mPa·s. Viscosity and vial neck diameter were shown to be the main contributors to the hold-up volume of sorbitol-based aqueous solutions in vial and withdrawal syringe. The hold-up volume of various molecules of therapeutic interest was successfully estimated using a model built from sorbitol-based aqueous solutions data. A total variability approach is proposed for estimating the overfill volume of liquid-in-vial drug products, taking into account the product hold-up volume in vial and withdrawal syringe, the filling variability, and the extractable volume test variability. This prediction model could provide a first guess of the fill volume range to be tested to support overfill volume definition.
Introduction
The most common container-closure system configuration for liquid and freeze-dried small-volume parenteral drug products is a glass vial, sealed with a rubber stopper and an aluminum crimp overseal (1⇓–3).
A slight volume excess—also referred to as overfill volume (Voverfill)—is allowed to ensure the label claim volume (Vlabel) can be extracted and administered (4⇓⇓⇓–8). The overfill volume should be minimized as much as possible to prevent unsafe handling (9) and limit drug product waste (10⇓⇓–13). Any overfill volume setting (target and range) needs to be justified, by referring to the maximum overfill volume proposed in USP <1151> (5), and based on product characteristics using extractable volume experimental data (9, 14).
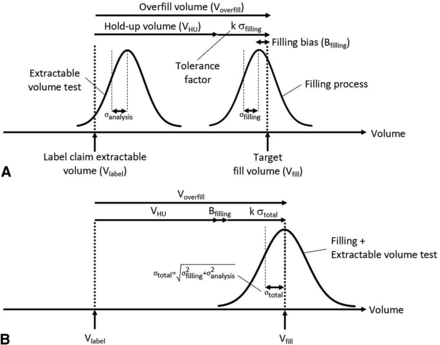
The overfill volume is recommended to be defined based on the hold-up volume in vial, withdrawal syringe, and needle (VHU), the filling line variation, and the extractable volume test method variability (14). A predictive modeling approach has been proposed recently to estimate VHU (15). In practice, Voverfill is sometimes defined as the sum of VHU and the filling process tolerance (16, 17), regardless of the extractable volume test method variability. The filling process tolerance is usually calculated as a multiple (k) of the fill volume standard deviation obtained from historical data (17⇓⇓–20) (Figure 1, Panel A).
State-of-the-art (A) and proposed (B) approaches for overfill volume definition.
Objective
The objective of this study was to evaluate if the overfill volume required for a liquid drug product in a vial can be estimated using a predictive modeling approach based on total variability (Figure 1, Panel B), taking into account the solution hold-up volume, filling variation, and extractable volume test variability.
Materials and Methods
Hold-Up Volume Determination
Materials:
D-Sorbitol (BioUltra ≥99.5%, cat. no. 85529) and Sterile Water for Injection (OmniPur®, cat. no. 4.86505) were purchased from Merck (Darmstadt, Germany). The following DIN ISO tubular Type I crimp glass vials (21) were purchased from Schott (Mainz, Germany): 2R (13 mm neck diameter, cat. no. 1096873), 6R (20 mm neck diameter, cat. no. 1123261), 10R (20 mm neck diameter, cat. no. 1096819), and 20R (20 mm neck diameter, cat. no. 1156521). FluroTec™-laminated 4023/50, B2-40, 13 mm (cat. no. 1358) and 20 mm (cat. no. 1343) bromobutyl serum stoppers were purchased from West Pharmaceutical Services (Exton, PA, United States); 13 mm (cat. no. 5209) and 20 mm (cat. no. 5115) diameter aluminum overseals with Flip-off® polypropylene disks also were obtained from West Pharmaceutical Services. The following polypropylene syringes were purchased from Becton Dickinson (Franklin Lakes, NJ, United States): 3 mL (cat. no. 309658), 5 mL (cat. no. 302187), 10 mL (cat. no. 300912), and 20 mL (cat. no. 300613) volume; 19 G x 1½” needles (cat. no. 301500) were also obtained from Becton Dickinson. The immunoglobulins G1 (IgG1) and G4 (IgG4), antigen-binding fragment (Fab'), PEGylated antigen-binding fragment (Fab'-PEG), single-domain (sdAb), bispecific (BsAb), and trispecific (TsAb) antibodies, peptide and small molecule drugs (SMD) are molecules of therapeutic interest manufactured by UCB Pharma (Braine-l'Alleud, Belgium).
Equipment:
Density (ρ, g/mL) measurements were performed at 20°C using a DM40 densitometer from Mettler Toledo (Columbus, OH, United States). Viscosity (η, mPa·s) measurements were performed at 20°C using a microVISC™ viscometer from RheoSense (San Ramon, CA, United States). Weight measurements (±0.001 g) were performed using an AX504 weighing scale from Mettler Toledo.
Vial Filling:
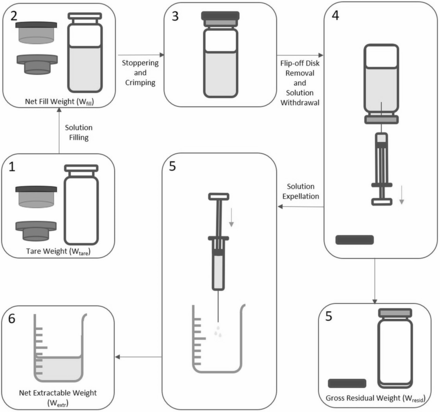
This hold-up volume determination study focused on three factors: viscosity (seven levels in the 1-40 mPa·s range), vial format (four levels: 2R, 6R, 10R, and 20R), and fill volume (five levels per vial format: 2R: 1.10, 1.20, 1.30, 1.40, and 1.50 mL; 6R: 3.20, 3.30, 3.40, 3.50, and 3.60 mL; 10R: 5.20, 5.35, 5.50, 5.65, and 5.80 mL; 20R: 10.20, 10.40, 10.60, 10.80, and 11.00 mL). A full factorial design approach (all combinations of factors and levels) was followed, leading to 140 experimental conditions. The tare weight (Wtare: vial, stopper, and overseal) was measured before filling (Figure 2). A minimum of two replicates per condition were prepared. Vials were filled by weight, taking into account the density of sorbi-tol solutions (Table I). The net fill weight (Wfill) was recorded. All vials were stoppered and crimped with aluminum overseals.
Vial filling and extractable volume testing.
Physical Properties of Sorbitol-Based Aqueous Solutions
Extractable Volume Testing:
The vials filled with sorbitol solutions were randomly allocated to eight analysts having some experience in extractable volume testing. The syringes were suitably sized to the volumes to be extracted: 2R vials – 3 mL syringes, 6R vials – 5 mL syringes, 10R vials – 10 mL syringes, and 20R vials – 20 mL syringes; 19 G × 1½” needles—wider than the 21 G needles of not less than 1” length recommended in compendial methods (4, 6⇓–8, 22)—were used to limit the withdrawal force required for viscous solutions during extractable volume testing. The syringe and needle were connected. The Flip-off® disk was removed and the vial stopper was pierced using the syringe needle. No air was expelled from the syringe into the vial to aid extraction. The entire contents of the inverted vial were manually extracted as far as possible in the syringe. The syringe needle was removed from the vial stopper. With the needle pointing upwards, the syringe was tapped to collapse any air bubbles. Air was carefully expelled from the syringe and needle until the first signs of liquid emerged from the needle tip. The syringe content was expelled (without emptying the needle) into a tared glass beaker. The net weight of expelled contents (Wextr) was recorded. The vial gross weight after solution withdrawal (Wresid, including Flip-off® disk weight) was recorded (Figure 2).
VHU was calculated using the following formula: VHU = (Wfill − Wextr)/ρ. The hold-up volume in vial (VHUv) was calculated using the following formula: VHUv = (Wresid − Wtare)/ρ. The hold-up volume in withdrawal syringe and needle (VHUs) was indirectly obtained by difference: VHUs = VHU − VHUv.
The effect of fill volume, solution viscosity, and vial format (fixed effects) and analyst (random effect) on VHU was analyzed using a mixed model (α = 0.05). A logarithmic transformation was applied to VHU and viscosity values. The extractable volume test method relative variability (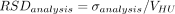 ) obtained by regression analysis (log-log model) (23) was defined as the combination of the vial-to-vial (model root mean square error) and analyst variabilities.
) obtained by regression analysis (log-log model) (23) was defined as the combination of the vial-to-vial (model root mean square error) and analyst variabilities.
Filling Process Variability Determination
The filling process variability was estimated using in-process fill weight values from 82 batches involving 17 liquid-in-vial drug product presentations (IgG1, IgG4, Fab', Fab'-PEG, BsAb, TsAb, or peptide) filled using a peristaltic pump. The viscosity, concentration, and fill volume ranged from 1–20 mPa·s, 1–160 mg/mL, and 1.0–16.8 mL, respectively.
The actual fill weight dependence on target fill weight (Vfill, fixed effect) and batch (random effect) was evaluated using a mixed model (α = 0.05). A logarithmic transformation was applied to actual and target fill weights. Two sources of filling variability were estimated (24) (Figure 1): average relative filling bias (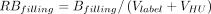 , root mean square of best linear unbiased predictors for batch effect—systematic error) and filling precision (
, root mean square of best linear unbiased predictors for batch effect—systematic error) and filling precision (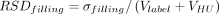 , model root mean square error, vial-to-vial variation—random error).
, model root mean square error, vial-to-vial variation—random error).
Total Variability Determination
Total variability was calculated as the root sum of squares of filling precision and extractable volume test method variability (25) (eq 1).
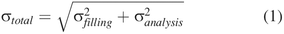
Data Analysis
Statistical analysis was performed using JMP 11.0.0 (SAS Institute, Cary, NC, United States). Graphs were created using Prism 8.1.1 (GraphPad Software, San Diego, CA, United States).
Results
First, the hold-up volume and filling process variability determination results are reported. Then a hold-up volume prediction model is proposed taking into account the solution viscosity and vial neck diameter. Finally, an overfill volume prediction model is proposed, based on the predicted hold-up volume, the filling process variability, and the extractable volume test method variability.
Hold-Up Volume Determination
Preparation of Sorbitol-Based Aqueous Solutions:
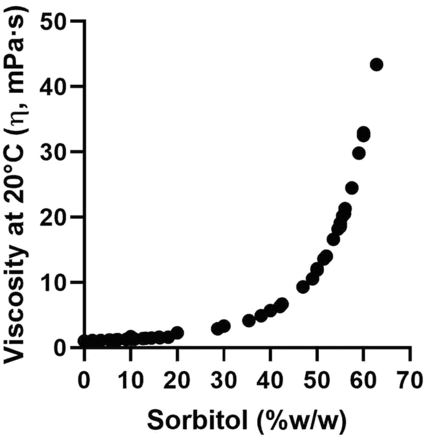
Literature (26, 27) and preliminary trials data show an exponential (28) increase of viscosity (at 20°C) with sorbitol concentration (Figure 3). Seven sorbitol-based aqueous solutions were prepared to cover a viscosity range of 1 to 40 mPa·s, which is typical of many parenteral formulations (28⇓–30) (Table I).
Evolution of viscosity with sorbitol concentration in water.
Extractable Volume Testing:
VHUv and VHU values were independent (P > 0.050) from the fill volume (15) and dependent (P < 0.001) on the solution viscosity and vial format (14, 15, 29). Post-hoc multiple comparisons of VHUv and VHU values per vial format using the Tukey-Kramer Honestly Significant Difference method (α = 0.05) identified two different groups among VHUv and VHU data: 13 mm (2R) and 20 mm (6R, 10R, and 20R) neck diameter vials. No significant impact (P > 0.050) of fill volume and solution viscosity on VHUs values was observed.
Summary results were calculated by averaging values from parameters for which no statistically significant impact on hold-up volume was found: hold-up volume results obtained across all fill volumes (n ≥ 10, i.e., five fill volumes at least in duplicate, Table II); VHUs average and standard deviation values obtained across all fill volumes and viscosities (n ≥ 87, i.e., five fill volumes and seven viscosities, at least in duplicate): 88 ± 35 µL (3 mL syringes), 91 ± 38 µL (5 mL syringes), 107 ± 44 µL (10 mL syringes), and 128 ± 51 µL (20 mL syringes).
Hold-up Volumes of Sorbitol-Based Aqueous Solutions, in Vial and Withdrawal Syringe. The Average and Standard Deviation Values Were Calculated from a Minimum of 10 Measurements (5 Fill Volumes Tested at Least in Duplicate)
The extractable volume test method variability (RSDanalysis) was calculated (Table III). The vial-to-vial (i.e., all other sources of variability than analyst: weighing scale, vial, stopper, syringe, and so forth) and analy-st contributions to RSDanalysis were 68% and 32%, respectively.
Filling Process and Extractable Volume Test Method Variabilities. 95% Confidence Intervals Are Reported into Parentheses
Filling Process Variability Determination
No significant impact of viscosity on filling variability was observed (P > 0.050). The in-process fill weight data were analyzed per vial neck diameter as a larger relative variability was observed for low fill volumes (19, 31). Filling precision (RSDfilling) and average relative filling biases (RBfilling) are reported in Table III. The batch-to-batch and vial-to-vial contributions to filling variability were, respectively, 23% and 77% (13 mm vials) and 47% and 53% (20 mm vials).
Hold-up Volume Prediction Model
A prediction model of VHU as a function of sorbitol viscosity (η, in mPa·s, at 20°C) was built (eq 2), in which 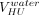 corresponds to the predicted hold-up volume of a solution having a viscosity of 1 mPa·s (close to water). The following
corresponds to the predicted hold-up volume of a solution having a viscosity of 1 mPa·s (close to water). The following 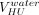 values were determined: 174 ± 11 µL for 13 mm vials and 263 ± 17 µL for 20 mm vials. The 1/7 exponent corresponds to a value of 0.14 ± 0.01.
values were determined: 174 ± 11 µL for 13 mm vials and 263 ± 17 µL for 20 mm vials. The 1/7 exponent corresponds to a value of 0.14 ± 0.01.
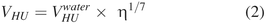
Although a narrow range of fill volumes was evaluated for each vial size, eq 2 was deemed applicable to any fill volume, as no statistically significant relationship between hold-up volume and fill volume was found.
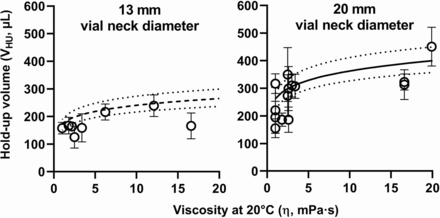
The predictability of eq 2 was evaluated in the 1-20 mPa·s viscosity range (Figure 4) using VHU results (Table IV) from several testing laboratories applying the multicompendial extractable volume test method (6⇓–8). This data set includes miscellaneous drug product presentations consisting of various active pharmaceutical ingredients, formulations, and primary packaging materials.
Hold-up volumes in vial and withdrawal syringe (VHU) of various drug product presentations (Table IV). Sorbitol-based VHU prediction models for 13 mm (dashed line) and 20 mm (plain line) vial neck diameter vials (eq 2) are presented, with their 95% confidence intervals (dotted lines). Error bars represent one standard deviation.
Hold-up Volumes (VHU) of Various Drug Product Presentations, in Vial and Withdrawal Syringe. The Average and Standard Deviation VHU Values Are Reported. Vial Format and Number of Measurements (n) Are Provided in Parentheses
Overfill Volume Prediction Model
The prediction modeling strategy proposed to evaluate the overfill volume of a liquid-in-vial drug product presentation is illustrated in Figure 1 (Panel B) and eq 3.
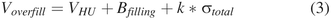
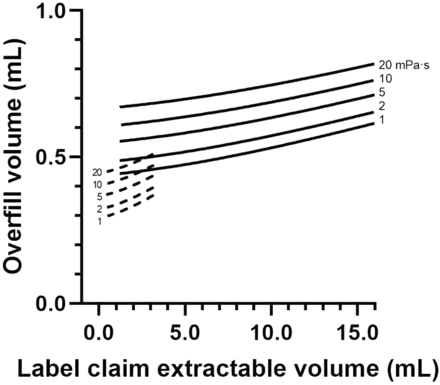
Figure 5 presents the predicted Voverfill values for a range of extractable volumes commonly used in 13 mm (2R to 4R) and 20 mm (6R to 20R) vials. The number of standard deviations (k) to ensure the extraction of a volume larger than or equal to Vlabel is defined as the 99.73% quantile of a standard normal distribution (k = z0.9973 = 2.78). A simpler model can be obtained by removing Bfilling from eq 3, as its contribution to Voverfill may be negligible (<1% of fill volume, see Table III).
Overfill volume prediction model (eq 3) for 13 mm (dashed line) and 20 mm (plain line) vial neck diameter vials, within a viscosity range of 1 to 20 mPa·s.
Discussion
A wide range of drug products are typically filled in small capacity vials (3, 13) below their nominal volume (3, 32, 33) to limit drug product wastage from partially used units (13). The overfill volume to ensure the label claim volume extraction from a small capacity vial could be larger than recommended in USP <1151> (5) and should be justified based on experimental data (9, 14). Overfill volume is traditionally defined by taking into account the hold-up volume in vial and withdrawal device (VHU) as well as the filling process tolerance (17⇓⇓–20). A predictive modeling approach may be of interest to provide a first estimation of the fill volume range to be tested to support overfill volume definition, especially at early stages of development in which drug substance material availability is often limited.
A VHU prediction model in vials (2R, 6R, and 10R) and syringes (1–10 mL) was proposed previously, based on extractable volume data from aqueous polyethylene glycol (PEG) 400 solutions in the 1–30 mPa·s viscosity range (15). This model predicts different VHU values for 6R and 10R vials, whereas our results suggest an identical VHUv/VHU value for 6R and 10R vials as they share the same stopper internal geometry (16).
The actual VHU values of most liquid-in-vial drug product presentations evaluated in this study (Table IV) lie within the VHU prediction model confidence interval (Figure 4). However, this model overestimates some actual VHU values (e.g., IgG4#6) and previously reported VHU results (15, 29), probably due to differences in solution surface tension because of formulation composition—for example, presence of surfactants (16, 34⇓–36), vial inner surface hydrophobicity (29), glass surface area or vial shoulder geometry (16), and withdrawal syringes or needles (37). Expelling air bubbles during extractable volume testing (6⇓–8) represents a risk of product loss that could lead to VHU overestimation, especially for products in which the full volume is injected, without preliminary air expulsion. The use of 19 G x 1½” needles to build the VHU prediction model may slightly contribute to overestimating VHU values generated using 21 G needles of not less than 2.5 cm (1”) long as currently requested by the multicompendial extractable volume test method (6⇓–8). VHU overestimation might lead to unsafe drug product handling (9), and VHU underestimation could possibly lead to failures in extractable volume testing. This emphasizes the need for experimentally verifying the predicted VHU value of a given drug product formulation or presentation (9, 14). The VHU prediction model presented in eq 2 and Figure 4 only applies to liquid-in-vial drug products. A similar approach could be followed to establish a VHU prediction model for other types of primary packaging (e.g., syringes, cartridges, and bottles).
The proposed overfill volume prediction model (eq 3) is based on a total variability approach, including both process (filling precision and bias) and analytical (extractable volume testing) variabilities. The vial filling process variability values (Table III) are in the range reported in the literature (0.25% to 1.0%) (19, 20, 38, 39). This methodology can be used for any type of primary packaging and allows the capture of all sources of variation to limit the risk of not meeting the extractable volume specification. In addition, this model may help in defining a maximum overfill volume value to avoid possible patient safety concerns (9).
Although extractable volume testing acceptance criteria can be met, the use of medical devices for product preparation and administration may require different vial overfill volumes (29, 40). Withdrawal syringes are typically quite variable (37), depending on hospital and country. Closed system transfer devices can have a hold-up volume of up to 1 mL (29). The only way to get a consistent hold-up volume is to co-package the vial with the withdrawal device, which is not always a viable option from a commercial standpoint.
Conclusion
A total variability approach to the overfill volume prediction of liquid-in-vial drug products is proposed, taking into account product viscosity, vial neck diameter, filling variability, and extractable volume test variability. This prediction model could provide a first guess of the volume range to be tested to support overfill volume definition.
Conflict of Interest Declaration
The authors declare no conflict of interest.
Acknowledgements
The authors thank Jérôme Beaufays, Ariane Descamps, Maxime Fouache, Albane Jacquet, Wesley Milton, and Anne Verlinden for technical support. Critical manuscript review by Olivier Dupont and statistical advice from Bianca Teodorescu are gratefully acknowledged.
- © PDA, Inc. 2022