Abstract
Adopting emerging microbiological methods is often desirable because it enables more advantageous, real-time monitoring practices. However, when the newer method measures contamination based on a different detection principle and provides results that are based on different units of measure, a paradigm shift is necessary. That shift can be one of the most difficult challenges in any such project and requires careful consideration. In this article, we explore the challenges presented by the bio-fluorescent particle counting (BFPC) technology, when considering that the traditional colony-forming unit (CFU) is the gold standard that any change is measured against. We examine why attempts to correlate newer units of measure used by biofluorescent particle counters, namely the auto-fluorescent units (AFUs), to the traditional CFUs are not necessarily appropriate. The article explores in depth why there is no consistent correlation factor between the two units of measure, and why that should not be a barrier to fully leveraging, implementing, and using such modern technologies in routine monitoring.
Preamble
Several industry working groups joined forces in 2021 to raise awareness and support the adoption of modern microbial methods. These groups include the BioPhorum Operations Group, the Kilmer Community Rapid Microbiology Methods group, the Online Water Bioburden Analyzer (OWBA) working group, and the Process and Environmental Monitoring Methods (PEMM) working group. This article is one of several published by the collaboration and part of a series on bio-fluorescent particle counters (BFPCs). The main article, “Challenges Encountered in the Implementation of Bio-Fluorescent Particle Counting Systems as a Routine Microbial Monitoring Tool” (https://journal.pda.org/content/early/2022/07/15/pdajpst.2021.012726), describes challenges encountered when implementing this modern technology. One challenge described in that paper is the nonequivalency of the colony-forming unit (CFU) with the auto-fluorescence unit (AFU). The present article addresses this topic in more detail.
Introduction
The challenges and protracted timelines for implementing modern microbial methods have been perceived as a barrier to innovation in the pharmaceutical industry. Although we have gained greater confidence to implement changes in the process steps, and even in the testing approach for nonmicrobial tests, there remains a real reluctance when the change involves a paradigm shift in microbial testing.
This reluctance starts with having to step away from our comfort zone of using traditional microbiological methods that have historically been the only option. Therefore, these methods are well-known even to nonmicrobiologists as the gold standard, which leads to the false assumption that any change must be measured against them. This expectation continues although the limitations of the traditional methods have long been well characterized (1).
Implementing newer microbiological methods can bring a wide range of benefits. For example, BFPCs offer real-time total and biological particle detection and counting. This approach could significantly improve environmental monitoring (EM) by detecting adverse trends early, responding to excursions in real-time and could also improve EM investigations root cause analyses by avoiding delays associated with obtaining traditional test results. Above all, it could significantly reduce contamination risks by eliminating human interventions required for manual sampling steps connected with the traditional methods.
The BFPC technology measures biological particle counts as AFUs. It does not rely on capture, growth, and counting of organisms as CFUs offered by traditional plate count methods, and therefore introduces a paradigm shift. Using the AFU versus the CFU is challenging, because the two units of measure have different signals and are not equivalent to each other.
As our manufacturing processes improve with the advances of science, so should our testing strategy, including microbial testing. For example, remote sampling using a BFPC would be most appropriate for aseptic processing in a gloveless isolator. This article discusses the fallacies and limitations associated with the CFU and why equivalency between the AFU and the CFU cannot be expected. This understanding will serve as a foundation to develop the right validation and implementation strategy, which will be discussed in future papers.
The Drawbacks of the CFU as a Gold Standard
Methods that provide results as CFUs are referred to as the gold standard when it comes to microbial testing in the pharmaceutical industry, with most regulatory documents providing acceptance criteria in units of CFU. For example, the Airborne Cleanliness Classification in the European Union (EU) current good manufacturing practices (cGMPs) for monitoring in ISO 5 (Grade A) through ISO 8 (Grade D) areas specifies Active Air Sampling Limits in CFU/m3 (2).
Whether the traditional plate count is used for EM or product testing, it is often incorrectly treated as an accurate quantification of any microbiological presence in the test sample. To understand why this is incorrect, we need to consider the method by which a result in CFUs is obtained. First, a sample is collected and processed in some way to get the endogenous microbial cells exposed to growth media. The media are then incubated at a specified temperature regimen for a specified duration. The cells must replicate to form colonies that are large enough for detection by the human eye or microcolonies detectable by an advanced imaging system within the incubation conditions selected. Growth of a microorganism is dependent on its nutritional, temperature, and oxygen requirements; if any of these are not met, insufficient growth will occur for visual detection. Even organisms that are expected to grow in the qualified conditions may not do so if they were collected from an environment that has left them weakened or stressed, or if they were killed during the collection and testing process. Their growth may also be inhibited due to the release of chemicals from neighboring microorganisms. Finally, the colonies that do form are often not the result of a single cell or spore but instead started from an aggregate that was composed of multiple cells or as multiple colonies closely located that grew into a single colony. Therefore, the CFU count is not an accurate measurement of the microbiological population present in the test sample. It is a measurement that is affected by a significant number of factors during the collection and growth process that can result in significant under-reporting (1).
Limitations of the traditional culture-based method have been recognized for over a century, with Winterberg (1898) reporting on the limitations of the CFU early on (3), and USP <1223> Validation of Alternative Microbiological Methods, directly citing that “traditional plate-count methods […] may recover 0.1%-1% of the actual microbial cells present in a sample” (4).
Therefore, it is important to acknowledge that all methods, even long-standing ones, are imperfect, and it is difficult at best to justify that one should be considered a gold standard against which the others are measured.
Overview of a Modern Technology That Reports in a Unit Not Equivalent to the CFU
BFPC enables active sampling and enumeration of both total and biological particles concurrently in the same sample. Continuous monitoring with BFPCs is thereby following the recommendations in the new EU Good Manufacturing Practice (GMP) Annex 1 (2). This methodology in turn eliminates manual sampling steps required for traditional EM, and with it, the associated contamination risks. When a BFPC is integrated into an isolator filling line, the method further eliminates the risks of missing samples due to operator error or to unintended damage or exposure of agar plates during sampling and collection.
Because sampling can be continuously performed during the setup and filling process, a greater volume of air is examined compared with the traditional active air monitoring methods that may be conducted as infrequently as once per shift. In addition, with a continuous monitoring method, every intervention and potential excursion occurring during aseptic processing can be proactively addressed, for example, by line stoppage or clearance, further increasing product quality and sterility assurance.
Real-time monitoring also allows for determination of an excursion as it occurs, which provides an opportunity to immediately react, remediate, and demonstrate a return to a controlled state. This is a huge advantage not available with traditional monitoring methods.
Finally, real-time BFPC monitoring is an excellent tool for tracing adverse trends, characterizing physical irregularities of the air handling systems, and eliminating potential contamination sources. For example, when applied to pharmaceutical purified water systems, the technology can be used to directly confirm the water quality used in cleaning or product manufacturing. In general, when using BFPCs either for air or water monitoring following startups or maintenance activities, production can be resumed more rapidly and with greater confidence than with traditional monitoring methods.
Why Are AFUs and CFUs Not Equivalent?
The BFPC technology measures biological particle counts as AFUs. Whereas most inert particles only scatter the light when hit by a laser, a particle that contains biomolecules like riboflavins and nicotinamide adenine dinucleotide hydrogen (NADH) also absorbs the light and emits it at longer wavelengths through a process known as laser-induced fluorescence (6). Because these biomolecules are ubiquitous in living cells, BFPCs can determine whether a particle is biological based on its characteristic fluorescence and report counts of total and biological particles. The biological particles detected over the sampling time are summed to obtain the AFU count per the volume sampled over that time.
Putting aside sampling variability, which will inevitably affect both the traditional and BFPC methods, there are several reasons why 1 CFU may not be equal to 1 AFU, and therefore why there is no consistent correlation factor assignable between both units of measurement.
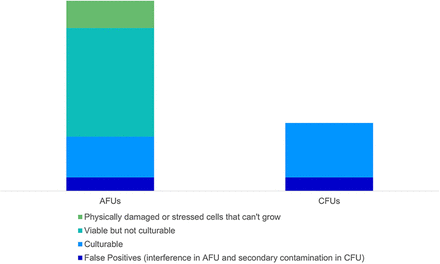
The particles present in a sample that could be counted or marked as a CFU and/or an AFU are illustrated in Figure 1. Note should be taken that the actual proportions of each category are not exact and will most likely vary in different facilities depending on the respective environmental conditions. We see in this illustration that AFUs may be composed of particles or organisms derived from up to four subgroups, and that these subgroups are not equally detected as CFUs.
A conceptualized visualization of what is counted as an AFU and as a CFU (relative sizes are for illustration purposes only and are subject to variation from sample to sample). AFU, auto-fluorescence unit; CFU, colony-forming unit.
For example, the category Viable but not Culturable (VBNC) includes dormant or stressed organisms that could potentially grow in better conditions, and those which simply cannot grow with the traditional nutrient media and incubation conditions (5). Therefore, in one experiment, a particular organism could generate a specific correlation factor between CFU and AFU, and the same organism could behave differently in another setting due to stress, for example.
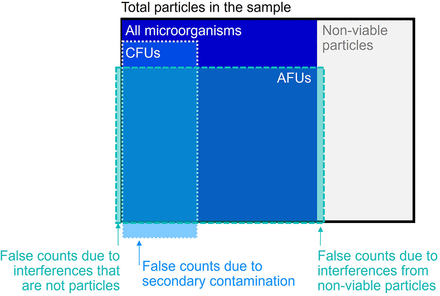
In general, it can be considered that a particle that yields a CFU would also generally yield 1 or more AFUs, but this is not an absolute; for example, when not all CFUs are counted because of particle size composition or orientation during measurement. There are also cases in which a particle that yields an AFU would not yield a CFU. Besides the often-cited case of VBNC being measured in AFU and not CFU, biomolecules from damaged cells might give individual AFU counts but not CFU counts. Other cases where 1 or more AFUs would not correlate to any microorganisms could occur, notably if an interferent is measured and incorrectly classified as a biological substance (false positive). There are nonbiologic particles, referred to as interferents, that can also fluoresce in the detection range of the BFPC system. BFPC systems use algorithms to identify the characteristic fluorescence of biologic particles, but some detection of interferent particles as biologic can occur. Mitigation of such interferent materials should be part of the method suitability testing performed with these systems. Other than that, there are no “false positives” expected on the AFU-level, because the risks for secondary contaminations connected with the traditional method are eliminated by the BFPC technology. However, as more particles have the potential to be counted as an AFU than as a CFU, the AFU count will generally exceed the CFU count. This is further conceptualized in Figure 2, where one can see where some particles may be both an AFU and a CFU, only one or the other, or neither. It also includes an illustration of potential false positives and false negatives.
A conceptualized visualization of the relationship between AFUs and CFUs (relative sizes are for illustration purposes only and are subject to variation from sample to sample). AFU, auto-fluorescence unit; CFU, colony-forming unit.
This illustrates that there is no specific correlation between the two units of measurement, especially in very clean environments where only a few particles are present. However, both are related, and AFU counts are commonly higher than CFU counts. This allows BFPCs to pick up signals from activities in the environment with more sensitivity and, therefore, more reliability.
Conclusion
The reluctance of the pharmaceutical industry to use BFPC-based analytical methods is understandable, as embracing this technology requires shattering some deep-rooted paradigms in pharmaceutical microbiology, given the long history of use of traditional methods that generate results as CFUs.
Regulatory guidelines, such as the EU GMP Annex 1 (2), have been encouraging the use of alternative technologies. However, these same guidance documents continue to include limits defined in terms of CFU. It is important to note that in the 2022 revision of EU GMP Annex 1, an encouraging step forward was taken in notes under Table II: Maximum Permitted Microbial Contamination Level During Qualification and Table VI: Maximum Action Limits for Viable Particle Contamination that reference use of different or new technologies that present results in a manner different from the CFU (2). These notes also mention an ability to establish different limits, based on the different unit of measure, with justification (2). BFPC and their mode of detection of microorganisms require a rethinking across the pharmaceutical industry for manufacturers, inspectors, and regulators to continue the paradigm shift from the traditional to modern monitoring methods.
With a deeper understanding of the limitations behind the CFU counts and why there can be no specific correlation between AFU and CFU, we will be able to establish appropriate validation strategies and acceptance criteria that will facilitate the adoption of BFPCs more widely. Proper understanding of the new method demonstrated performance and appropriately set limits will enable better, more controlled processes and enhance the assurance of quality for the benefit of patients.
Additional publications in this series are planned to discuss proposed validation strategies in more detail and to address the determination of baseline AFU counts to establish alert and action levels for air and water monitoring.
Conflict of Interest Declaration
The working group collaboration of authors includes vendors, end users, and consultant members. Each author’s affiliation is provided in the list of authors.
The authors declare that they have no competing interests.
- © PDA, Inc. 2023