Abstract
The results of a proof-of-principle study demonstrating a new analytical technique for detecting microbial growth directly in pharmaceutical containers are described. This analytical technique, laser-based headspace analysis, uses tunable diode laser absorption spectroscopy to nondestructively determine gas concentrations in the headspace of a media-filled pharmaceutical container. For detecting microbial growth, the levels of headspace oxygen and carbon dioxide are measured. Once aerobic microorganisms begin to divide after the lag phase and enter the exponential growth phase, there will be significant consumption of oxygen and concomitant production of carbon dioxide in the sealed container. Laser-based headspace analysis can accurately measure these changes in the headspace gas composition. The carbon dioxide and oxygen measurement data for the representative microorganisms Staphylococcus aureus, Bacillus subtilis, Candida albicans, and Aspergillus brasiliensis were modeled using the Baranyi-Roberts equation. The mathematical modeling allowed quantitative comparisons to be made between the data from the different microorganisms as well as to the known growth curves based on microbial count. Because laser-based headspace analysis is noninvasive and can be automated to analyze the headspace of pharmaceutical containers at inspection speeds of several hundred containers per minute on-line, some potential new applications are enabled. These include replacing the current manual human visual inspection with an automated analytical inspection machine to determine microbial contamination of media fill and pharmaceutical drug product vials.
LAY ABSTRACT: A novel analytical technique has been demonstrated for detecting microbial growth in media-filled pharmaceutical containers. This analytical technique, laser-based headspace analysis, uses tunable diode laser absorption spectroscopy to determine gas concentrations in the headspace of a pharmaceutical container. For detecting microbial growth, the levels of headspace oxygen and carbon dioxide are measured. The study shows that once aerobic microorganisms begin to grow after the lag phase and enter the exponential growth phase there will be a significant consumption of oxygen in the sealed container as well as a corresponding production of carbon dioxide. Headspace analysis can accurately measure and monitor these changes in the headspace gas composition and could therefore be used to detect contaminated pharmaceutical containers. Because the technique can be automated to analyze hundreds of containers a minute on-line, there are opportunities for implementing a headspace inspection machine to perform automated inspection of media fills used to validate aseptic filling operations.
- Microbial detection
- Microbiological test method
- Media fill inspection
- Headspace analysis
- Headspace oxygen
- Headspace carbon dioxide
- Microbial growth
- Frequency modulation spectroscopy
Introduction
It was recently demonstrated that microbial growth in media-filled vials could be detected by monitoring the consumption of oxygen in the vial headspace (1). This was accomplished by using a tunable diode laser absorption spectroscopy (TDLAS) method. The diode laser spectroscopy technique, frequency modulation spectroscopy (FMS), is a powerful analytical technique that can achieve a high signal-to-noise ratio enabling the measurement of trace amounts of gases (2). Over the past decade this analytical method has been implemented in the pharmaceutical industry for noninvasive measurements of headspace gas composition and headspace pressure/vacuum levels in pharmaceutical drug containers. Previous publications have described the use of FMS to characterize the headspace in sterile pharmaceutical product vials for the confirmation of vacuum or inert gases, container-closure integrity, and moisture content of lyophilized products (3⇓⇓⇓⇓–8). One of the applications has been to integrate the laser-based headspace sensors into automated inspection machine platforms for the on-line inspection of finished pharmaceutical product (9). The method is also described as a deterministic container closure integrity test method in the revised USP <1207> Sterile Product Packaging—Integrity Evaluation recently released for public comment (10). The results described in this report show that nondestructive, laser-based headspace analysis can detect microbial growth in stoppered and sealed pharmaceutical vials filled with microbiological growth media by monitoring headspace oxygen consumption and corresponding carbon dioxide production. Using this approach, the determination of microbial growth can be measured analytically directly in pharmaceutical containers in a nondestructive manner. Any contamination, whether by aerobic microorganisms, anaerobic microorganisms as well as mycoplasamas, that causes detectable changes in the headspace gas composition of the sealed container will be detected. The primary proof-of-principle study described demonstrates using headspace analysis to detect media vials contaminated with five representative compendial aerobic microorganisms. Additional secondary studies also described in this paper demonstrate the ability of headspace analysis to detect media vials contaminated with aerobic or anaerobic microorganisms and mycoplasmas. Because headspace analysis can be automated at inspection speeds of several hundred vials per minute on-line, a potential application is pharmaceutical aerobic media fill inspections as part of aseptic filling process validation. This represents an opportunity for replacing the labor intensive human visual inspection process (a subjective method) with an automated analytical inspection for media fills. This change would improve the reliability of media fill inspection, reduce the media fill inspection time and the required human labor, and improve vial reconciliation and data integrity. In addition, headspace analysis could potentially be used to determine the lag time and growth rate of microorganisms in microbial challenges for beyond-use dating studies and for the nondestructive verification of presumptive sterility test failures. A presumptive sterility test failure could be confirmed by performing headspace analysis of the sterility test vials or canisters to check for headspace oxygen consumption and/or carbon dioxide production in the canister due to microbial growth prior to subculturing the sterility test media. Finally, the measured headspace oxygen and carbon dioxide data curves can be analyzed with mathematical modeling. The results of the primary study were used to demonstrate that this mathematical approach enables the differences in the gaseous exchange characteristics from microorganism to microorganism to be quantified as well as to investigate if the headspace gas curves mimic bacterial growth curves.
Media Fill Validation for Aseptic Filling Processes
To validate an aseptic filling operation, it is pharmaceutical industry practice to fill, stopper, and seal containers used for product with tryptic soy broth (TSB), incorporating likely interventions within the media fill to mimic the standard filling process. The number of media-filled containers could be in excess of 10,000 depending on the typical product batch size. Media fill incubation conditions can vary but a common practice is to incubate the containers at 20–25 °C for 7 days, followed by a further 7 days at 30–35 °C. The containers are then visually inspected under the supervision of a trained microbiologist. Filled units lacking container-closure integrity may be rejected prior to incubation. A 100% inventory control of the media fill containers is expected by the regulators. After incubation, the vials are visually inspected for microbial contamination in the form of turbidity, pellicle formation, floccular-suspended material, and/or precipitation. In general, a single turbid, filled container after incubation, irrespective of the batch size, will trigger an investigation, while two or more turbid, filled containers require a repeat of the media fill. The contents of the so-called turbid vials are microscopically examined and subcultured to confirm the presence of microorganisms and the microbial isolates identified to the species level to aid investigation (11, 12).
Microbial Metabolism as Related to Media Fills:
Microorganisms can be conveniently classified into aerobic, facultative anaerobic and anaerobic organisms. For example, obligate aerobes cannot grow in the absence of oxygen or other electron acceptors such as nitrate and lack a functional fermentative metabolism. These organisms convert glucose to carbon dioxide using the Embeden-Meyerhof-Parnas (EMP) pathway or glycolysis, the Krebs or citric acid cycle and the electron transport chain to obtain energy in the form of ATP with oxygen being the terminal electron acceptor (13). The overall reaction can be represented as C6H12O6 + 6O2 → 6CO2 + 6H2O + 38ATP. With aerobic metabolism one would expect to find in the headspace of a media fill vial evidence of a stoichiometric oxygen consumption and carbon dioxide generation.
In contrast, facultative and obligate anaerobes grow in either the presence or absence of oxygen, respectively, (in the absence of oxygen they use fermentative pathways or anaerobic respiration) so their patterns of oxygen consumption and carbon dioxide production in a media fill vial will be more complex. They contain two groups, either cytochrome independent, (e.g. lactic acid bacteria), or cytochrome dependent (e.g., member of the family Enterobacteriaea). The various fermentative pathways of microorganisms in these two groups will produce end products that include lactic acid, alcohol, a mixture of organic acids (acetic, lactic, and formic acid), carbon dioxide, ethanol, and butanediol (13). It is therefore more difficult to predict exactly what the headspace gas dynamics will be as the various microorganisms begin to grow. As a media fill vial is a closed system, oxygen will be limited. The TSB, if saturated with oxygen, will contain less than 8 mg of oxygen per liter and the headspace will contain around 270 mg/L. It is possible that soon into the exponential growth phase the oxygen will be depleted with the resulting pattern of metabolism shifting from aerobic to anaerobic.
Oxygen Consumption
A recent publication reported the lag phase oxygen consumption per colony-forming unit (CFU) (Qo2) for Escherichia coli under nutritional-limited growth as 2.4 × 10–7 mol O2 CFU–1 day–1 or 1 × 10–8 mol O2 CFU–1 h–1 (14). The oxygen depletion in a pharmaceutical vial due to exponential microbial growth will be detected by headspace analysis. It is assumed that this type of oxygen depletion directly links to the microbial count for aerobic growth. Growth of facultative anaerobes would also be detected through oxygen depletion in the sealed vial, but the correlation to microbial count could be more complicated. In both cases, exponential microbial growth will also be identified by carbon dioxide production in the headspace.
Media Fill Broth
(a) TSB (Soybean-Casein Digest Broth):
The composition of the compendial TSB is given in Table I.
The Ingredients, Composition, and Function of the Components of TSB
What may be lacking for the broadest aerobic microbial growth in TSB includes essential minerals, some vitamins, and fatty acids. Furthermore, an important point for this study is that traditional microbiological growth media are optimized for microbial growth identified by turbidity of the media and not by headspace analysis. It might be that microbiological growth media, as well as incubation conditions, could be optimized for maximum change in the headspace oxygen and/or carbon dioxide levels to identify microbial growth.
In a static, batch culture like a media fill vial, a typical bacterial growth curve will consist of five distinct phases, namely, the lag, exponential growth, stationary, death, and long-term stationary phases. It has been assumed that the lag phase allows for the adaptation of the bacterial cells to the new culture conditions and may include repair to cellular damage and synthesis of enzymes needed to enter the exponential growth phase. The most rapid oxygen consumption and carbon dioxide production will be delayed until the microorganism enters the mid-exponential growth phase where there will be a sufficient number of respiring microbial cells to result in significant change to the headspace composition.
(b) Fluid Thioglycollate Medium (FTM):
It is standard practice for pharmaceutical media fills to use TSB. However, to enhance the growth of anaerobes when they have been implicated in sterility test failures or for sterile products with a nitrogen blanket in their headspace, FTM is usually used. The composition of the compendial FTM is given in Table II.
The Ingredients, Composition, and Function of the Components of FTM
The top one-third of the medium will be oxygenated with the redox indicator resazurin appearing red, and will support the growth of facultative anaerobes and strict aerobes, while the remaining two-thirds will support facultative aerobes and strict anaerobes. Under one of the experimental conditions used in this study, where the medium-filled vial is purged with nitrogen, the entire medium will be anaerobic. Ingredients that may be lacking in FTM for full-range anaerobic growth are hemin and vitamin K.
Although FTM is suitable for sterility testing as it contains neutralizers for mercuric preservative and supports the growth of aerobic as well as facultative and obligate anaerobes, it is not used to isolate anaerobes in a clinical setting.
Growth of Mycoplasma in Media Fills
Members of the order Mycoplasmatales are flexible, pleomorphoric organisms as small as 0.2 micron that lack a cell wall and are commonly known as contaminants of mammalian cell cultures. As they have a small genome (600–1400 kB) compared to the 4.64 mB of E. coli K-12, they have a reduced synthetic capability and rely on host or cell-culture metabolic products such as fatty acids, sterols, and other complex lipids for growth. Growth media containing fetal calf serum, cholesterol, and yeast extract are widely used to isolate mycoplasma from cell culture, vaccines, and other biologics. However, mycoplasma are a widely diverse group of facultative anaerobic microorganisms with a broad host range including humans, other animals, insects and plants, as well as different metabolic patterns so that the headspace dynamics are difficult to predict.
Mycoplasma became, in addition to a cell culture contaminant, a compliance concern when a major biotechnology company observed multiple media fill validation failures due to Acholeplasma laidlawii contamination. Turbid media, when subcultured and subjected to microscopic examination, initially failed to implicate a microrganism. The contamination was eventually identified as due to A. Laidlawi using 16S ribosomal RNA (rRNA) base sequencing. Further investigation showed that the batches of dehydrated TSB used were contaminated with the mycoplasma, which were not removed from the media fill by sterile filtration using a 0.2 micron sterilizing-grade filter. This was mitigated either by using a 0.1 micron sterilizing-grade filter, purchasing irradiated media, or moving from animal- to plant-based media. The latter was ultimately unsuccessful as the plant-based material was also contaminated by mycoplasma (15).
Baranyi-Roberts Equation Application to the CO2 and O2 Measurements:
The microbial headspace oxygen and carbon dioxide measurement curves over time were modeled using the Baranyi–Robert equation shown below (16). While this equation is normally used to model microbial growth measured by plate count or turbidity, the sigmoidal shape of the microbial CO2 production curves and adjusted O2 consumption curves suggested it would be a candidate for modeling the headspace gas curves.
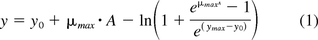
In its standard use, the variable y represents the microbial count in log10 CFU mL–1, with y0 representing the initial bacterial population count, and ymax representing the final bacterial population count. For the purposes of this study, the variable y was adapted to represent the log10 gas concentrations, with y0 and ymax signifying the initial and final log10 headspace gas concentrations. The two remaining parameters of the equation are μmax, the maximum specific growth rate (h–1), and h0, the bacterial adaptability factor (relating to the initial physiological state of the microorganism being examined), with A being defined as
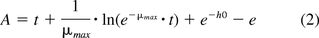
The lag time is related to both h0 and μmax by

The adjusted O2 consumption curves were generated by using the magnitude of the difference in O2 levels from point to point to construct a sigmoidal curve that increases according to the magnitude of O2 consumption in the original curve. This changes the orientation of the curve to be similar to the CO2 production curve thereby enabling application of the Baranyi-Robert equation for modeling the O2 results.
Materials and Methods
Media Fill and Inoculated Vial Preparation
In the primary experiment, the 10 mL borosilicate glass vials were sterilized, aseptically filled with approximately 5 mL of TSB, (Becton, Dickinson and Company, Franklin Lakes, NJ), stoppered, and capped in a clean room located at Afton Scientific (Charlottesville, VA). After a 2 week incubation period to confirm sterility, the vials were inoculated with <100 CFU of five representative challenge microorganisms using commercially prepared cultures as per the instructions of the manufacturer (EZ-CFU, Microbiologics, Inc., St Cloud, MN). The five challenge microorganisms represent compendia-specified microorganisms (USP Chapters <61>, <62>, and <71>).
The flip-tops of the caps were removed and the vials were injected with the different inocula (Table III) via sterile insulin syringes. Self-sealing rubber stoppers were used to maintain container-closure integrity after injection. Seven sample sets were prepared including a baseline control, a negative control inoculated with sterile air, and five different challenge microorganisms. Each set consisted of twenty 10 mL vials.
The Seven Sets Each with Different Inocula Prepared in This Study
Incubation Conditions
The media fill vials were stored in an environmental chamber for the course of the 14 day study. The samples were incubated at 20–25 °C for the first 7 days and 30–35 °C for the following 7 days. The samples were removed from the environmental chamber shortly before visual inspection and headspace measurement sessions to come to ambient temperature. Table IV lists the general description, typical optimum growth temperature and the appearance of exponential growth in TSB for each of the challenge organisms.
Description of Primary Challenge Organisms
Visual Inspection
The vials were visually inspected and assessed for the presence or absence of microbial growth daily by qualified laboratory technicians. The visual conditions of the samples were then recorded and later compared with the headspace analysis measurements. It should be noted that without continuous monitoring of the vials it is not possible to determine exactly when turbidity occurred in the vials in relationship to the headspace changes.
Oxygen Headspace Analysis
Headspace oxygen measurements were performed using a nondestructive headspace oxygen analyzer (Model FMS-760, Lighthouse Instruments, Charlottesville, VA). Analyzer calibration was performed using certified 20% and 0% oxygen standards. National Institute of Standards and Technology (NIST)-traceable certified reference standards were used to evaluate system performance (accuracy, precision, linearity, and limit of detection) of the FMS-760 and to verify system calibration.
The certified standards were made by backfilling 10 mL vials with certified NIST-traceable oxygen mixtures and then flame sealing them. The standards were made with glass vials identical to those used in the media fill. Table V shows the results of repeated measurements of the known oxygen standards over the course of the study and demonstrates the accuracy and precision of the headspace oxygen measurements for the 10 mL vial configuration. Before every measurement session, one measurement was taken of each of the six known oxygen standards to verify the performance of the system. The headspace oxygen concentration in each sample vial was then measured and recorded. Each sample vial was measured once. The first vial in each set was measured five consecutive times each day to determine measurement accuracy on the sample vials.
Results of Measurements on Certified Standards of Known Oxygen Concentration throughout the 14 Day Study
Carbon Dioxide Headspace Analysis
Headspace carbon dioxide measurements were performed using a nondestructive headspace carbon dioxide analyzer (Model FMS-CO2, Lighthouse Instruments, Charlottesville, VA). Calibration was performed using a certified carbon dioxide standard. Certified reference standards were used to evaluate system performance (accuracy, precision, linearity, and limit of detection) of the FMS-CO2 and verify system calibration.
The certified standards were made by backfilling 10 mL vials with certified NIST-traceable carbon dioxide gas and then flame sealing them. The standards were made with glass vials identical to those used in the media fill. Table VI shows the results of repeated measurements of the known carbon dioxide standards over the course of the study that demonstrates the accuracy and precision of the headspace carbon dioxide measurements for the 10 mL vial configuration.
Results of Measurements on Certified Standards of Known Carbon Dioxide Gas Pressure in Units of torr throughout the 14 Day Study
Carbon dioxide measurements are reported in units of torr. However, because media fill vials are at near-atmospheric pressure, it is possible to convert those measurements to units of % atm. This allows for easy comparison of the carbon dioxide and oxygen measurements of the media fill vials.
One measurement was taken of each of the four known carbon dioxide standards to verify the performance of the system. The headspace carbon dioxide in each sample vial was then measured and recorded. Each sample vial was measured once. The first vial in each set was measured five consecutive times each day to determine measurement accuracy, that is, system suitability on the sample vials.
Chemical Oxidation of the TSB
Prior to any microbial growth, a difference was observed between the expected initial headspace oxygen (20.9% atm) and measured oxygen concentrations in the media fill vials. This was due to two factors: spectroscopic broadening of the oxygen signal due to moisture in the headspace and chemical oxidation of the broth. To quantify the signal broadening, five vials were filled with water and five with fresh TSB with all vials having an atmospheric air headspace. The headspace oxygen and carbon dioxide levels in every sample were measured and montiored over the course of the 2 week incubation period. In addition, the headspace oxygen levels of the original baseline and negative control samples were monitored over the course of 3 months.
Secondary Studies
As described above, the primary proof-of-principle experiment focused on detecting the growth of aerobic microorganisms inoculated into vials filled with TSB. Secondary measurements were also done using headspace analysis to demonstrate the growth of anaerobes and mycoplasmas. For anaerobes, FTM was used and the media vials were purged with nitrogen so that facultative and strict anaerobes—but not aerobes—would grow in the FTM. A total of 2 mL of FTM medium was added to a standard VCDIN2R vial. The vials were then inoculated with the appropriate microorganism to a final concentration of 10 CFU/mL. To ensure initial headspace oxygen levels were below 1% atm, the vials were again purged for 30 seconds with nitrogen directly after inoculation. Triplicate samples were inoculated with the corresponding microorganism and were incubated at a temperature of 30–35 °C. The generation of headspace carbon dioxide was then followed over a period of more than 8 days. Table VII lists the general description, typical optimum-growth temperature, and the appearance of exponential growth in FTM for each of the challenge organisms.
Anaerobes Used in the Secondary Study
For the detection of mycoplasmas, media-filled vials were prepared with basic broth or Z+ broth (Microsafe Inc., Leiden, The Netherlands) depending on the microorganism being tested. Similarly, a total of 2 mL of growth media was added to a VCDIN2R vial. Triplicate samples were inoculated with the mycoplasma organisms and incubated at a temperature of 35–37 °C. The broth was proprietary but would be based on pleuropneumonia-like organism (PPLO/mycoplasma) broth supplemented with yeast extract and horse serum. The headspace oxygen consumption and headspace carbon dioxide production curves were then monitored over a 12 day incubation time.
Finally, a proof-of-principle experiment was performed to investigate if contamination of actual pharmaceutical product could be measured with headspace analysis. Vials of commercial pharmaceutical human albumin, 5%, were sourced and then inoculated with eight standard test microorganisms. Headspace oxygen and carbon dioxide levels were monitored during a 14 day incubation (20–25 °C for 7 days, followed by a further 7 days at 30–35 °C).
Results and Discussion
Experimental Data
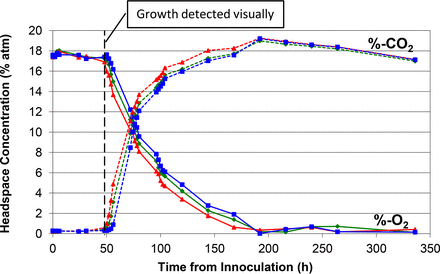
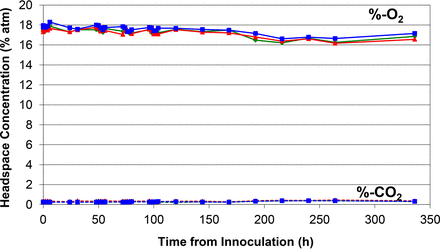
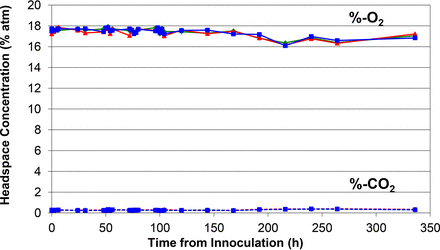
Time plots of the measured headspace oxygen and carbon dioxide levels in the media vials and the time point when visual microbial growth was first observed during the incubation period are shown in Figures 1 to 7.
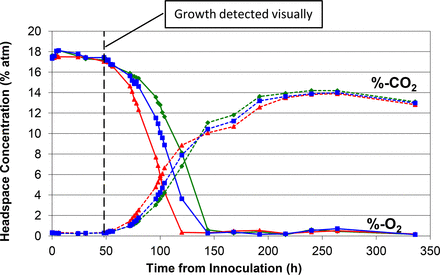
Measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in media vials innoculated with A. brasiliensis. The time point at which growth was detected visually is indicated by the vertical dotted line. The results of three samples are plotted to indicate the spread between extreme high, medium, and low measurement results.
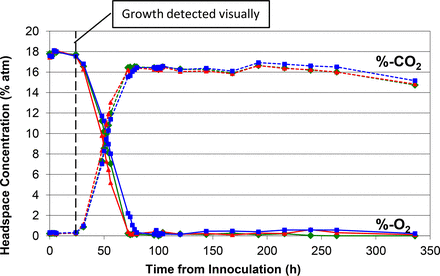
Similar plot of measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in media vials innoculated with B. spizizenii.
Similar plot of measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in media vials innoculated with C. albicans.
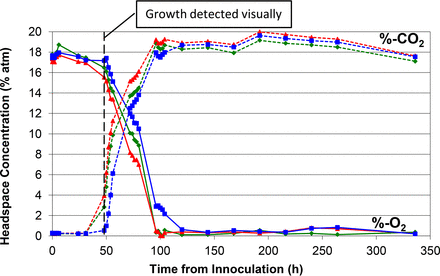
Similar plot of measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in media vials innoculated with P. aeruginosa.
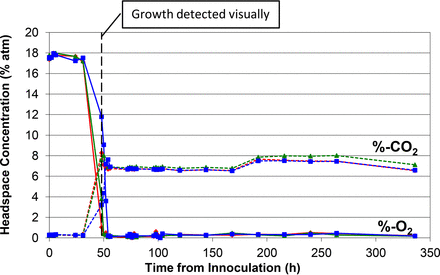
Similar plot of measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in media vials innoculated with S. aureus.
Similar plot of measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in the negative control media vials.
Similar plot of measured headspace oxygen and carbon dioxide levels over the 14 day incubation period in the baseline control vials.
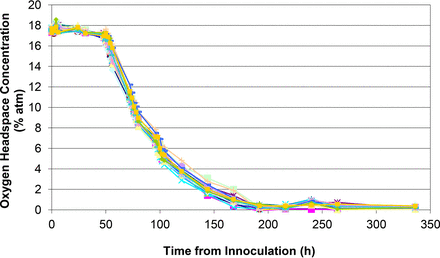
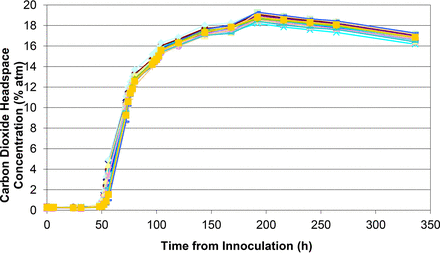
Figures 1 to 5 show the results from the five standard test organisms. Figures 6 and 7 show the results from the negative control set and the baseline control set, respectively. The graphs show the oxygen consumption and carbon dioxide production in the headspace of three samples from each of the test organisms from the time of inoculation of the media vial through the 14 day media fill incubation period. For each microorganism, three samples from the set of 20 were chosen to be plotted and represent the extreme high sample, extreme low sample, and a middle sample in terms of headspace oxygen and carbon dioxide levels. The graphs in Figures 1 to 5, therefore, illustrate how consistent the headspace oxygen consumption and carbon dioxide production were across the sample set of 20 media vials for each microorganism. The error bars for the measured data points can be deduced from the documented headspace analyzer performance in Table VII and Table VIII, and are smaller than the data points themselves in the graphs in Figures 1 to 7. Although the visual observations were not continuous, it is notable that microbial growth was visually observed just prior to, or early in the period of rapid change in headspace composition. Figures 8 and 9 show the results from all twenty samples inoculated with the bacterium S. aureus which clearly demonstrate the consistency of the measured oxygen consumption and carbon dioxide production curves across the set of 20 samples.
Mycoplasma Used in the Secondary Study
Measured headspace oxygen levels over the 14 day incubation period for all 20 media-filled vials innoculated with S. aureus.
Measured headspace carbon dioxde levels over the 14 day incubation period for all 20 media-filled vials innoculated with S. aureus.
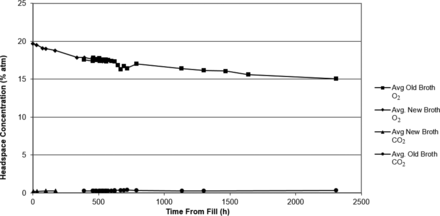
The results of both the negative control set and the baseline control in Figures 6 and 7, respectively, show a slight decrease in the headspace oxygen levels and no carbon dioxide production over the incubation period (336 h). The slight decrease in headspace oxygen levels is due to TSB auto-oxidation. The TSB auto-oxidation was confirmed with a separate media study. Five vials were filled with a fresh batch of TSB and five vials were filled with water. Initial headspace oxygen measurements of both sets gave very similar readings of ∼19.8% atm, implying that the aforementioned water vapor broadening of the headspace-oxygen signal in a TSB vial (see section Chemical Oxidation of the TSB) is similar to that for water. In other words, at atmospheric levels, the initial headspace oxygen content in a TSB media vial is underestimated by ∼1.1% atm. The new broth vials proceeded to chemically oxidize and the oxygen levels dropped to ∼17.9% atm in 2 weeks, matching what was observed in the study control vials 2 weeks after fill. This explains the initial headspace oxygen levels ∼17.9% in the study results, as the starting point of the inoculation study was 2 weeks after filling of the vials with TSB. The oxygen levels in the water vials stayed constant. The oxidation behavior of the TSB media was studied in more detail by monitoring headspace oxygen as well as carbon dioxide levels in the study control vials over a period of 100 days (2400 h). The results are plotted in Figure 10.
Measured headspace oxygen and carbon dioxide levels in TSB media vials over a period of 100 days (2400 h).
Experimental Data Secondary Studies
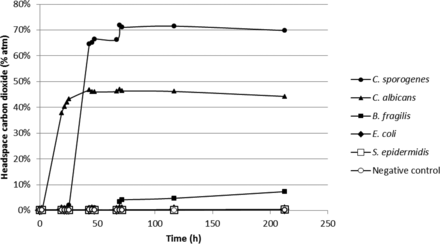
In addition to the results of the principal study just described, secondary data was also generated to investigate the possibility of detecting the growth of anaerobes and mycoplasmas with noninvasive laser-based headspace analysis. As described in the Materials and Methods section, standard VCDIN2R vials were filled with 2 mL of FTM for the anaerobe experiment. The headspace of each vial was purged with nitrogen to create anaerobic conditions. The initial headspace oxygen levels were near zero and did not increase during the incubation. The generation of headspace carbon dioxide was followed over an incubation period of 9 days at a temperature of 30–35 °C (Figure 11). The strict anaerobe C. sporogenes has the greatest measurable CO2 production and B. fragilis the least. The growth of B. fragilis is reported to be stimulated by the presence of hemin and vitamin K which, as stated earlier, are not present in FTM. The reason for the absence of growth indicated by zero CO2 production for E. coli and Staphylococcus epidermidis is not obvious to the authors but may be related to rendering the FTM fully anaerobic with 100% nitrogen in the headspace. It should be noted that the media vials in this study inoculated with E. coli and S. epidermidis did not display any turbidity and that these samples were not subcultured to evaluate the remaining microbial count after incubation. In general, the results demonstrate the possibility of detecting the growth of some anaerobic organisms by monitoring the production of carbon dioxide in the headspace.
Measured headspace carbon dioxide levels over an incubation period of 9 days in nitrogen-purged FTM medium-filled vials innoculated with different (facultative) anaerobic organisms. The plotted values are the mean of measurements taken on three replicate vials.
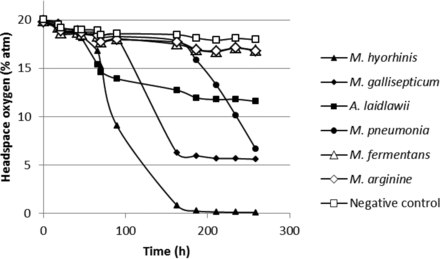
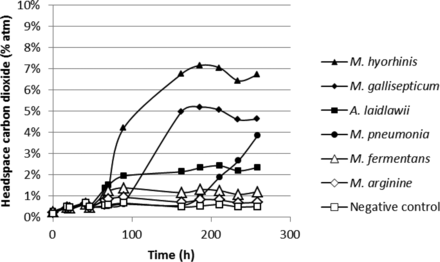
Figures 12 and 13 show the headspace oxygen consumption and headspace carbon dioxide production curves, respectively, over a 12 day incubation period at a temperature of 35–37 °C for the mycoplasma study. In this study, a proprietary growth media was used. Without a detailed knowledge of the medium composition and metabolic patterns of the different species of mycoplasma, the authors cannot comment on the headspace changes. However, from the results graphed in Figures 12 and 13, it is demonstrated that the headspace oxygen and carbon dioxide measurements were able to detect growth of some mycoplasmas. It should be noted that the media vials in this study that did not show clear changes in the headspace gas content also did not display any discoloration of the proprietary growth media.
Measured headspace oxygen levels over an incubation period of 12 days in media-filled vials innoculated with different mycoplasmas. The plotted values are from a single sample vial.
Measured headspace carbon dioxde levels over an incubation period of 12 days in media-filled vials innoculated with different mycoplasmas. The plotted values are from a single sample vial.
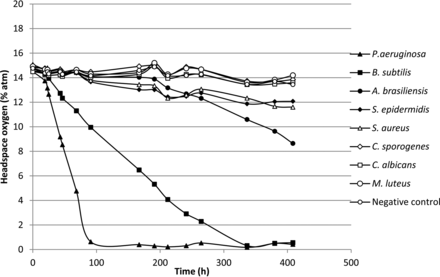
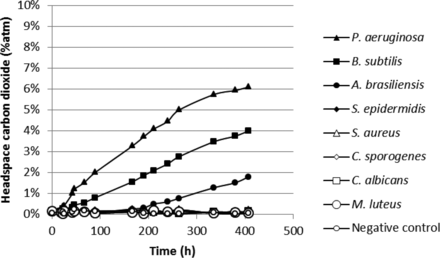
Finally, measurements were also made on vials of commercial pharmaceutical human albumin, which is known to support microbial growth. The human albumin product vials were inoculated with standard test microorganisms. Also in this case, growth of organisms was detected with laser-based headspace analysis during a 14 day incubation (20–25 °C for 7 days, followed by a further 7 days at 30–35 °C) as shown in Figures 14 and 15. Unlike a microbiological culture medium, 5% human-albumin solution is not optimized for microbial growth. It is noteable that the two strict aerobes P. aeruginosa and B. subtilis, which are rich in proteolytic enzymes and not reliant on vitamins and growth factors, grew most abundantly while the strict anaerobe C. sporogenes grew least abundantly.
Measured headspace oxygen levels over an incubation period of 14 days in pharmaceutical human albumin product vials innoculated with different microorganisms.
Measured headspace carbon dioxide levels over an incubation period of 14 days in pharmaceutical human albumin product vials innoculated with different microorganisms.
Discussion of the Experimental Results
The results of the primary study demonstrated that a nondestructive headspace gas measurement can identify growth in TSB media vials after the vials were inoculated with five representative challenge microrganisms. The headspace measurements could not only identify the onset of exponential growth in the contaminated media vials, but could robustly follow the growth evolution in the samples throughout the incubation period. During the writing of this paper, the authors learned that these results have subsequently been confirmed at other pharmaceutical microbiology laboratories (17, 18). These recent preliminary studies have also demonstrated that, in addition to the five representative organisms used in this study, headspace analysis can also detect the growth of typical house isolates that have been found in sterile manufacturing facilities (17). These results are promising indications that headspace analysis could be used for an analytical inspection of media fills. Undoubtedly, replacing the labor intensive and subjective human visual inspection process with an automated analytical headspace inspection for media fills will require formal method development and validation studies prior to implementation. Formal method development and validation would include a robust demonstration that headspace analysis identifies media fill vials that are contaminated with a representative set of microorganisms including house isolates that are of interest for a specific sterile manufacturing facility. For each microorganism type and container configuration, it should be demonstrated that a headspace carbon dioxide and/or oxygen measurement distinguishes a contaminated vial from one that is sterile. The results from method development and validation studies should define headspace carbon dioxide and/or headspace oxygen limits that clearly distinguish all contaminated vials from sterile vials after a defined incubation. A headspace inspection process can then be defined and qualified to robustly demonstrate that headspace measurements can be made with sufficient accuracy and precision in the inspection process to detect all contaminated media vials. Future studies could investigate the possibility of using headspace analysis to inspect media fills subjected to varying incubation conditions such as single temperature or shorter incubation times.
In addition to the primary application described in this paper of using headspace analysis for media fill inspection, there are other potentially interesting applications for using headspace in microbiological activities. The ability to make headspace measurements directly in sterile pharmaceutical containers raises the additional opportunity of using headspace analysis for sterility troubleshooting in actual pharmaceutical product. For example, for product that is growth promoting (such as human albumin), a measurement of elevated carbon dioxide levels could indicate a potentially contaminated container. Other potential applications envisioned by the authors include using headspace analysis to determine the lag time and growth rate of microorganisms (including anaerobes and mycoplasmas) in microbial challenges for in-use dating studies, for the nondestructive verification of presumptive sterility test failures, and for growth media optimization studies. Each of these applications could be enabled by using nondestructive headspace analysis in containers or sterility test vials or canisters to quanitatively measure and monitor changing carbon dioxide and/or oxygen levels resulting from microbial growth.
Mathematical Modeling of the O2 Consumption and CO2 Production Data
In the previous section, a general approach was described for method development and validation of headspace analysis to detect a contaminated media-filled unit in a media fill inspection process. It is assumed that the headspace gas dynamics in a sealed media vial correlate to actual microbial growth. It is, therefore, interesting to investigate if the measured headspace dynamics of oxygen consumption and carbon dioxide production reflect microbial growth curves based on microbial counts. A better understanding of how headspace gas dynamics correlate to microbial growth can perhaps expand the possible utility of headspace analysis to microbiological applications such as the ones mentioned at the end of the previous section.
In order to model the results of the primary proof-of-principle study described in this paper, the Baranyi-Roberts equation (16) was applied to fit a growth curve to both the carbon dioxide and modified oxygen data points for each microorganism being studied. The results from the mathematical modeling could then be compared to microbial growth parameters known from microbial count studies. In addition, the modeling enabled the quantification of differences in the gas curves from microorganism to microorganism. Modeling the results of the five representative organisms required frequent data points in the exponential growth phase. All of the data collected in the study was robust enough for the mathematical modeling except for the data collected for P. aeruginosa. The extremely steep change of the headspace carbon dioxide and oxygen curves for this microorganism in the exponential growth phase (see Figure 4) meant that too few data points were available for the mathematical modeling. The model calculations determined the maximum growth rate, generation time, and apparent lag phase duration for each microorganism, which are the three most meaningful growth parameters.
Discussion of the Carbon Dioxide Data Modeling
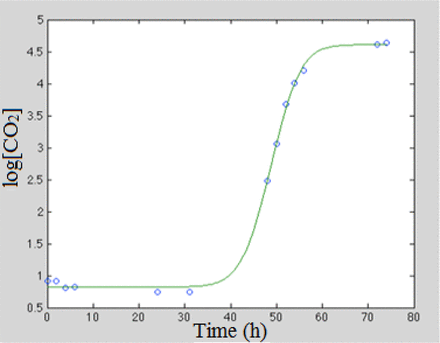
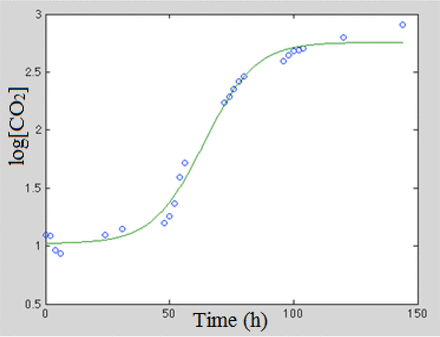
It was possible to obtain good quality fits to the carbon dioxide production curves by varying the parameters of the Baranyi-Roberts equation using regression analysis with the obtained fits to the gas curves of all four microorganisms having average R2 values above 0.99. An example result is shown in Figure 16 which plots the carbon dioxide production curve for one of the S. aureus samples along with the corresponding fit using the Baranyi-Robert equation.
Example of a measured CO2 growth curve, represented by the log10 headspace carbon dioxide concentrations, for TSB sample vial 134 innoculated with the bacterium S. aureus along with the fit obtained using the Baranyi-Robert equation.
Fitting the carbon dioxide gas curves measured in each sample for each microorganism resulted in the calculated growth parameters summarized in Table IX. From a sensitivity analysis of the Baranyi-Roberts regression model used for the fitting, it could be concluded that the standard deviations (SDs) seen in the calculated growth parameters from sample to sample of a specific microorganism are probably due to natural variation rather than any regression-related issues.
CO2 Results Summary
Discussion of the Oxygen Data Modeling
After providing the adjustments discussed previously to the original oxygen consumption data, the Baranyi-Roberts equation was successfully applied to fitting the resulting modified oxygen data. With the high R2 value as an indication (approximately 0.999 on average for all data sets), the quality of the fits proved to be as good or better than for the carbon dioxide data. An example result is shown in Figure 17, which plots the modified oxygen gas curve for the same S. aureus sample along with the corresponding fit using the Baranyi-Robert equation.
Example of a modified O2 growth curve, represented by the log10 headspace oxygen concentrations for sample 134 of the bacterium S. Aureus.
Fitting the oxygen gas curves measured in each sample for each microorganism resulted in the calculated growth parameters summarized in Table X. Again, from the sensitivity analysis of the Baranyi-Roberts regression model, the deviations seen in the calculated growth parameters from sample to sample are most likely the result of natural variation rather than errors in the regression process.
O2 Results Summary
Oxygen and Carbon Dioxide Modeling Comparison
The objective of the mathematical modeling was twofold: (1) to gain insight into whether or not the measured headspace gas dynamics reflect actual microbial growth, and (2) to quantify the measured gas curves so that they could be compared with one another. Progress toward meeting the first objective can be made by comparing the calculated average lag times (represented by λ) with the duration of the lag phase reported by other researchers from studies determining microbial counts (19⇓–21). In general, the lag times calculated from the headspace gas curves are longer than those seen during in-use dating studies with sterile pharmaceutical products, that is, on the order of 6 h (22). Upon consideration, this is not surprising. One could theorize that microorganisms begin to grow by first consuming the available oxygen dissolved in the TSB media before accessing the oxygen in the headspace. In such a situation, headspace gas dynamics would lag microbial count. It is also apparent from the gas curves in Figures 1⇑⇑⇑–5 that there is not a one-to-one conversion of oxygen molecules to carbon dioxide molecules in the headspace as would be expected from the aforementioned respiration equation: C6H12O6 + 6O2 → 6CO2 + 6H2O + 38 ATP. It is therefore resonable to conclude that the headspace dynamics are, in fact, a reflection of the specific metabolic pathways of each microorganism. Detection of microbial growth using headspace analysis is therefore accomplished by measuring the results of the metabolic process rather than direct microbial count or evidence of microbial growth as turbidity. A detailed discussion of the relationship between microbial metabolism and the gaseous exchange patterns for a particular microrganism is outside the scope of this article. However, it is straightforward, as demonstrated here, to generate analytical headspace gas data that correlates to microbial growth dynamics in a quantitative model. It is suggested by the authors that future work could investigate this approach in more detail. The growth parameters of the Baranyi-Roberts equation could be redefined for headspace analysis—that is, lag phase could be defined as gas-lag phase, maximum growth rate could be defined as maximum gas consumption/production rate, and so on. In addition, studies could be done to investigate the correlation between gas dynamics and actual microbial count.
Finally, it can be concluded from the mathematical modeling that it is possible to make a thorough quantitative analysis of the headspace gas dynamics measured during microbial growth. This in turn enables detailed growth comparisons between different samples of the same microorganism, between different microorganisms, and between different conditions (growth media, incubation conditions). Using the Baranyi-Roberts equation, the gas curves can be described by quantitative parameters that include a gas-lag phase and a maximum oxygen consumption rate or a maximum carbon dioxide production rate. The ability to straightforwardly collect analytical headspace gas data coupled with the ability to perform a thorough quantitative analysis of the headspace gas curves measured during microbial growth is a powerful combination that could be applied in studies aimed at the optimization of growth media and incubation conditions per microorganism.
Conclusions
Laser-based headspace analysis is a promising technique for performing nondestructive, growth-based microbial detection directly in pharmaceutical containers. The nondestructive nature of the measurement and the fact that it can be automated for high-throughput measurements are unique advantages that enable potentially interesting applications. Chief among these is automated media fill inspection as the drastic changes in the headspace composition of a contaminated media vial during a standard incubation is robustly detected by laser-based headspace analysis. Other potential applications include confirmation of presumptive sterility test failures, and possible sterility troubleshooting of actual pharmaceutical product batches. The headspace oxygen and carbon dioxide curves measured to indicate microbial growth can be analyzed with mathematical modeling. This approach enables the differences in the gaseous exchange characteristics from microorganism to microorganism to be quantified and is potentially useful for calculating relative lag phase and maximum growth rate in terms of the headspace dynamics. The ease with which headspace gas curves can be measured nondestructively, coupled with robust quantitative analysis, enables an approach for optimizing media and incubation time and temperature conditions for microbial detection using headspace analysis.
Conflict of Interest Declaration
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank staff at Afton Scientific for support in preparation and incubation of the media vials for the primary study. For the studies involving aenerobic organisms, mycoplasma, and human albumin product, the authors would like to thank Nigel Stapleton at Microsafe for fruitful discussion, as well as the staff at Microsafe for support in preparation and incubation of the media vials.
- © PDA, Inc. 2016